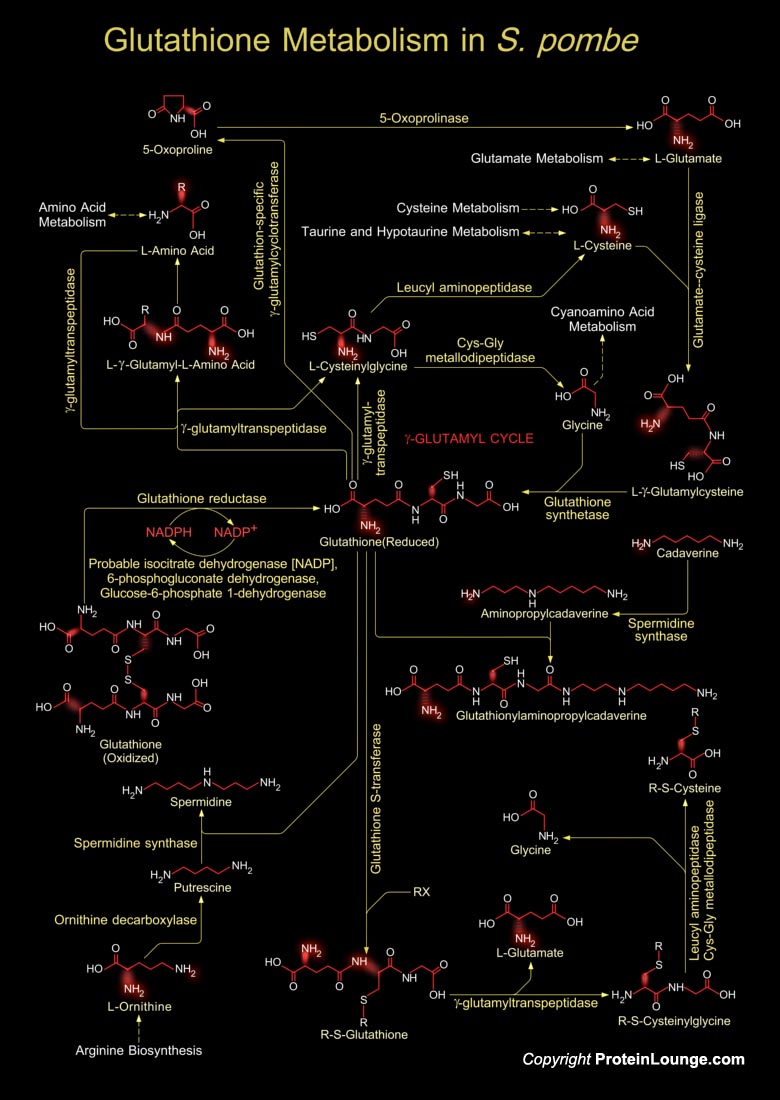
Glutathione is a sulfhydryl (-SH) antioxidant, antitoxin, and enzyme cofactor. It is ubiquitous in animals, plants, and microorganisms, and being water soluble is found mainly in the cell cytosol and other aqueous phases of the living system. It has been assigned several cellular functions, including protection against oxidative damage, maintenance of a reducing cellular thiol-disulfide balance, electron donation for a number of enzymes, protection of protein sulfhydryls from irreversible oxidation, and detoxification of foreign compounds. Glutathione is synthesized enzymatically from its constituent amino acids in two consecutive reactions. GSA1 (Glutathione Synthetase or GSH2) catalyzes the second step and it occurs in two different forms in Schizosaccharomyces[..]
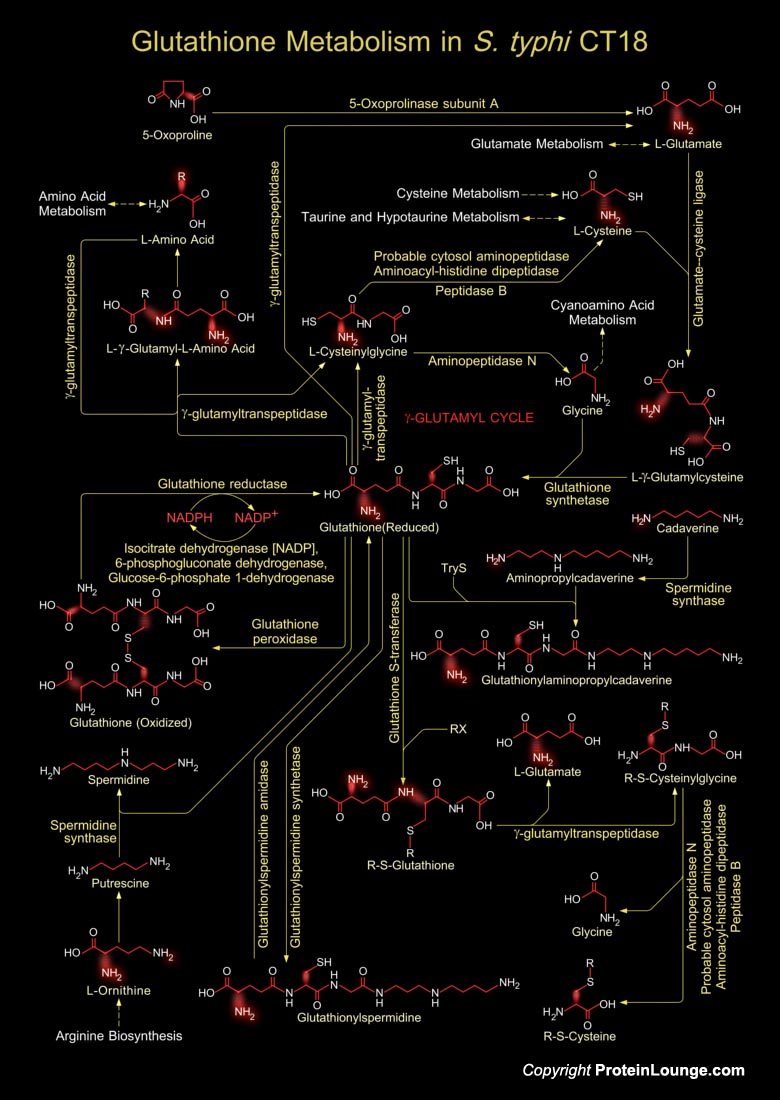
Glutathione is a sulfhydryl (-SH) antioxidant, antitoxin, and enzyme cofactor. It is ubiquitous in animals, plants, and microorganisms, and being water soluble is found mainly in the cell cytosol and other aqueous phases of the living system. It cannot enter most cells directly and therefore must be made available inside the cell from its three constituent amino acids: Glycine, Glutamate and Cysteine. The rate at which glutathione can be made depends on the availability of Cysteine, which is relatively scarce in foodstuffs. It often attains millimolar levels inside cells, which makes it one of the most highly concentrated intracellular antioxidants. Glutathione exists in two forms. The antioxidant "reduced Glutathione" tripeptide is conventionally called[..]
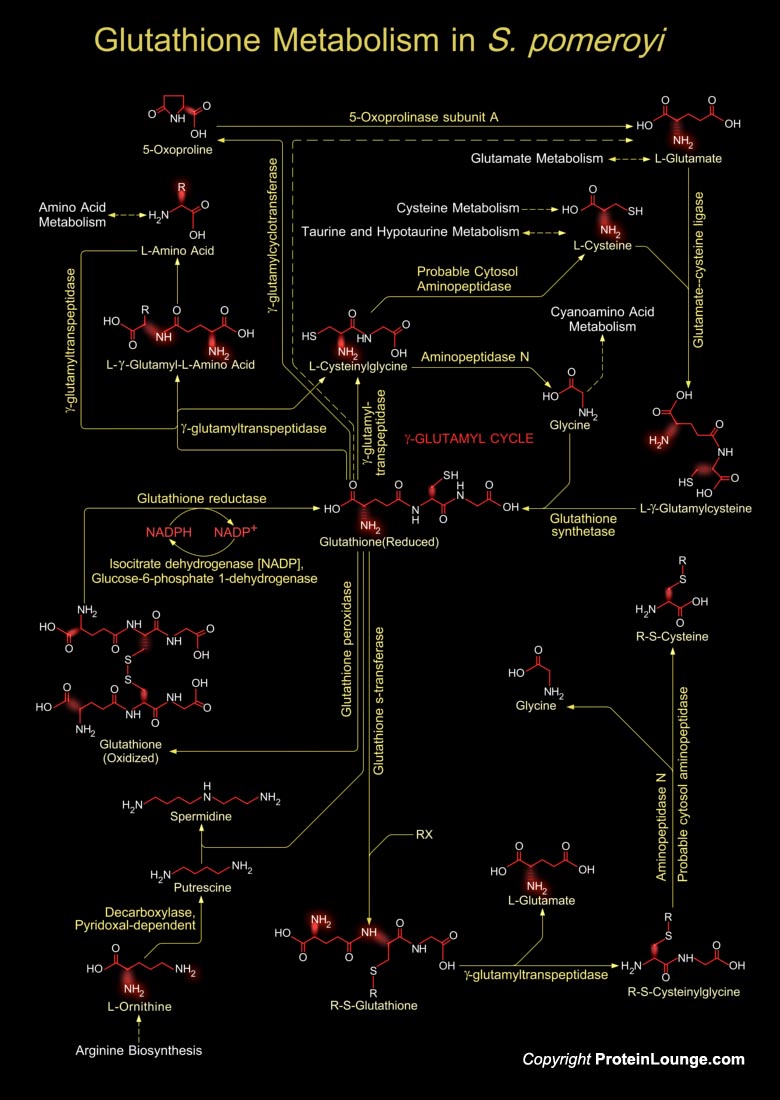
Glutathione is a sulfhydryl (-SH) antioxidant, antitoxin, and enzyme cofactor. It is ubiquitous in animals, plants, and microorganisms, and being water soluble is found mainly in the cell cytosol and other aqueous phases of the living system. Glutathione is a tripeptide composed of Glutamate, Cysteine and Glycine that has numerous important functions within cells. It is homeostatically controlled, both inside the cell and outside and often attains millimolar levels inside cells, which makes it one of the most highly concentrated intracellular antioxidants. Glutathione exists in two forms. The antioxidant "reduced Glutathione" tripeptide is conventionally called Glutathione and abbreviated Gsh; the oxidized form is a sulfur-sulfur linked compound, known as[..]
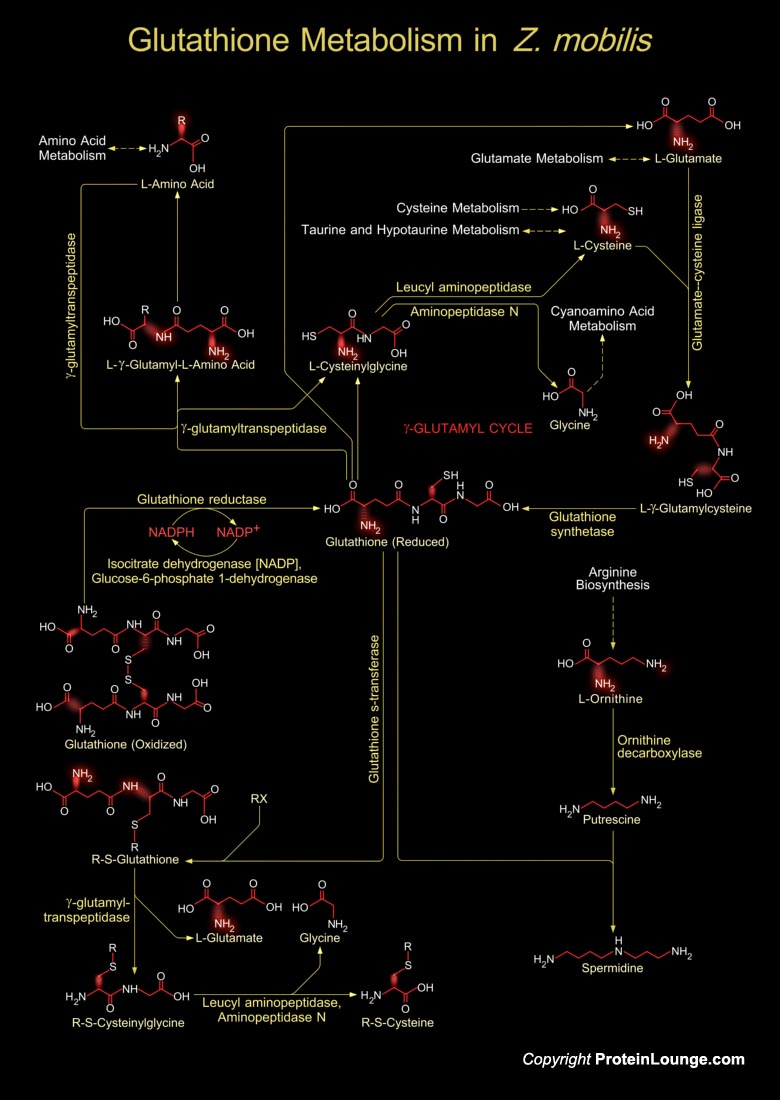
Zymomonas mobilis is an ethanologenic microorganism used for the production of fuel ethanol (Ref.1). Glutathione metabolism in Z. mobilis involves both the synthesis of Glutathione and its catabolism. Glutathione is a small molecule found in almost every cell. It cannot enter most cells directly and therefore must be made available inside the cell from its three constituent amino acids: Glycine, Glutamate and Cysteine. The rate at which Glutathione can be made depends on the availability of Cysteine, which is relatively scarce in foodstuffs. Furthermore, the Cysteine molecule has a sulfur-containing portion which gives the whole Glutathione molecule its ‘biochemical activity’, i.e. its ability to carry out the vitally important functions. Cysteine can also[..]
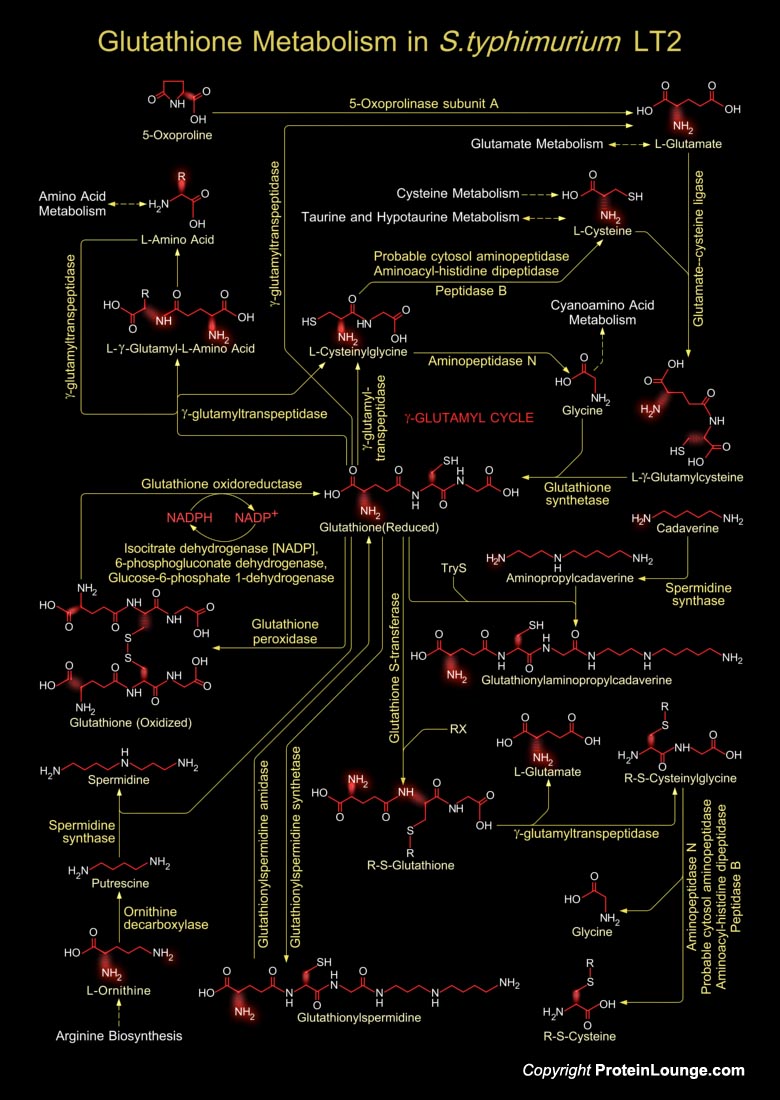
Glutathione is a sulfhydryl (-SH) antioxidant, antitoxin, and enzyme cofactor. It is ubiquitous in animals, plants, and microorganisms, and being water soluble is found mainly in the cell cytosol and other aqueous phases of the living system. It cannot enter most cells directly and therefore must be made available inside the cell from its three constituent amino acids: Glycine, Glutamate and Cysteine. The rate at which glutathione can be made depends on the availability of Cysteine, which is relatively scarce in foodstuffs. Furthermore, the Cysteine molecule has a sulfur-containing portion which gives the whole Glutathione molecule its ‘biochemical activity’. Cysteine can also enter the Glutathione metabolism through several other metabolic pathways like[..]
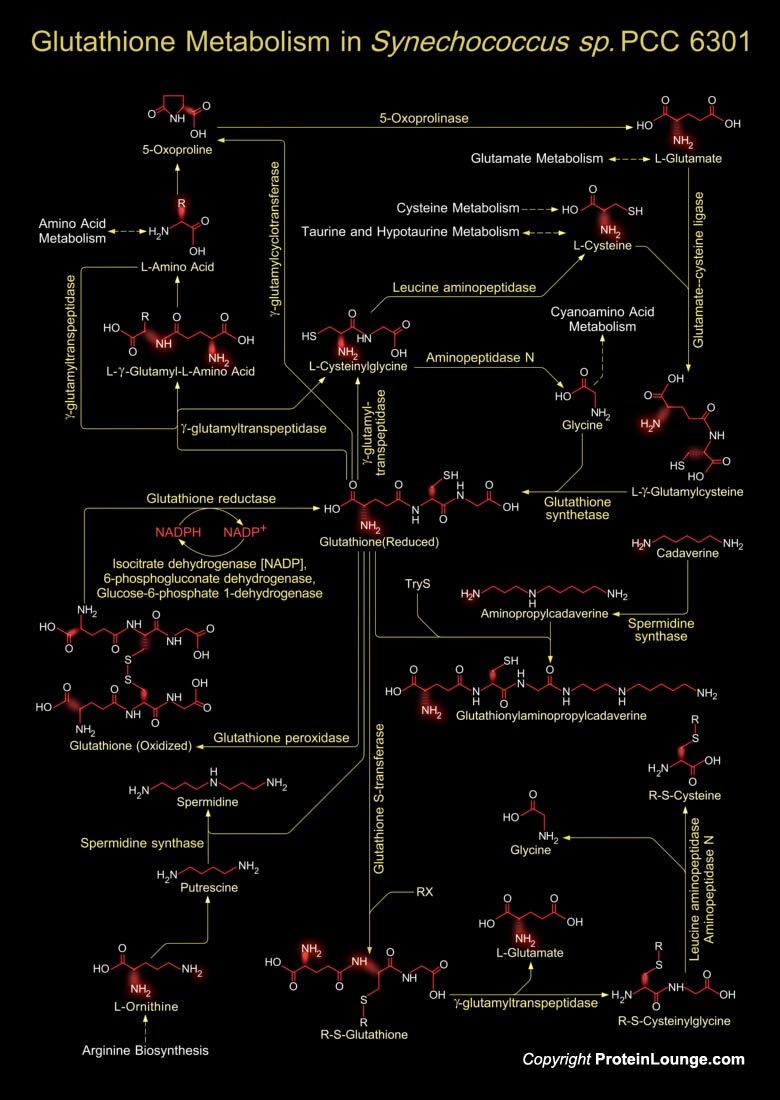
Marine unicellular Cyanobacteria of the Synechococcus group occupy an important position at the base of the marine food web. They are abundant in the world's oceans and as a result are major primary producers on a global scale and one of the most numerous genomes on earth. They have the ability to acquire major nutrients and trace metals at the submicromolar concentrations found in the oligotrophic open seas, and their light-harvesting apparatus is uniquely adapted to the spectral quality of light in the ocean (Ref.1). Glutathione metabolism in Synechococcus sp. involves both the synthesis of Glutathione and its catabolism. Glutathione is a small molecule found in almost every cell. It cannot enter most cells directly and therefore must be made available inside the[..]
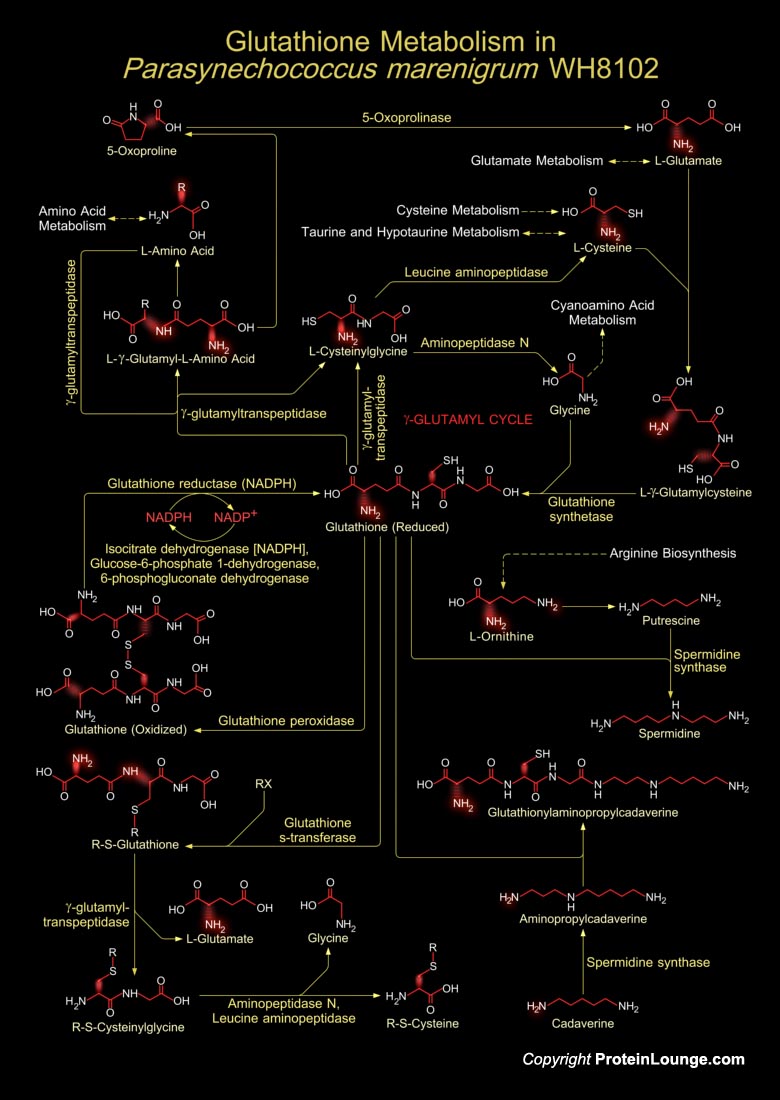
Marine unicellular Cyanobacteria of the Synechococcus group occupy an important position at the base of the marine food web. They are abundant in the world's oceans and as a result are major primary producers on a global scale and one of the most numerous genomes on earth Synechococcus is the main source of primary productin in oligotrophic, pelagic waters. Many genera of Cyanobacteria thrive in nutrient-rich, eutrophic environments, and are, as such considered indicators of eutrophication, which currently troubles many aquatic ecosystems. Synechococcus sp. strain WH8102 is a motile strain that can be grown in both natural and artificial seawater liquid media as well as on plates and is amenable to biochemical and genetic manipulation. It dwells in the most[..]
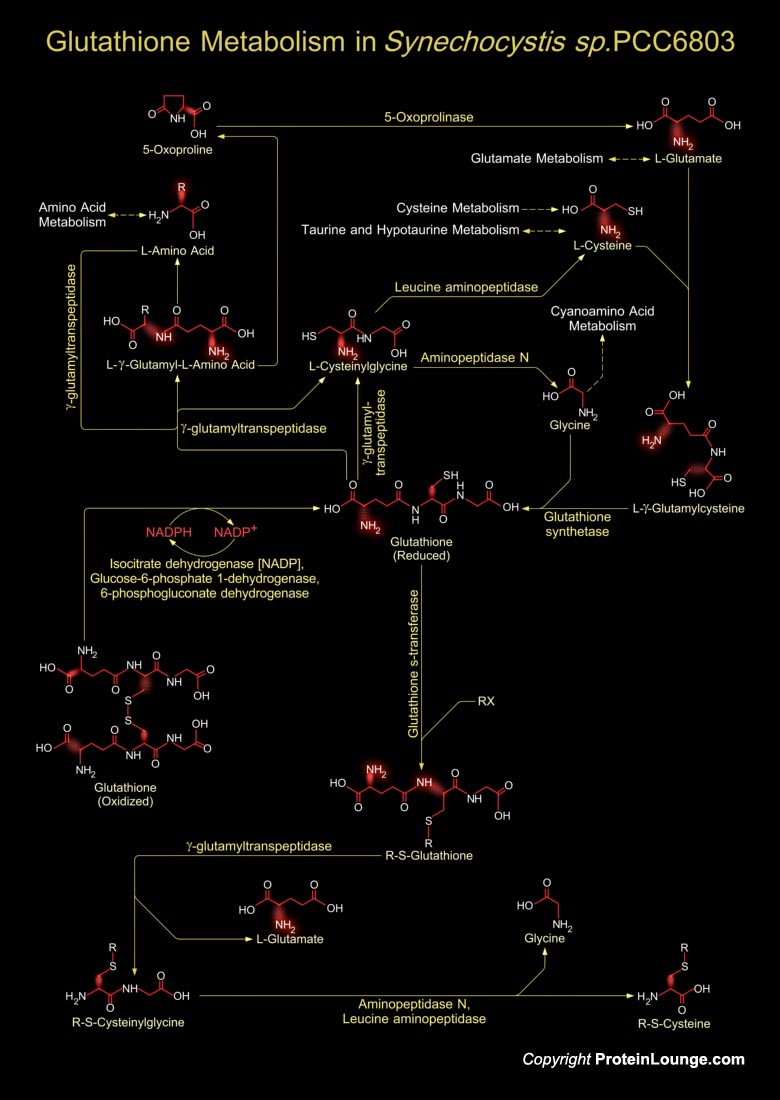
Synechocystiae are unicellular, photoautotrophic, facultative glucose-heterotrophic bacteria. They are oxygenic photosynthetic with two photosystems at their disposal, similar to those in algae and plants, and they can fix nitrogen. Synechocystis sp. PCC6803 can grow in the absence of photosynthesis if a suitable fixed-carbon source such as glucose is provided. The total length of Synechocystis sp. strain PCC6803 is 3,573,470 bp and it has developed into a Cyanobacterium model. Glutathione metabolism in Synechocystis sp. involves both the synthesis of Glutathione and its catabolism. Glutathione is a small molecule found in almost every cell. It cannot enter most cells directly and therefore must be made available inside the cell from its three constituent amino acids:[..]
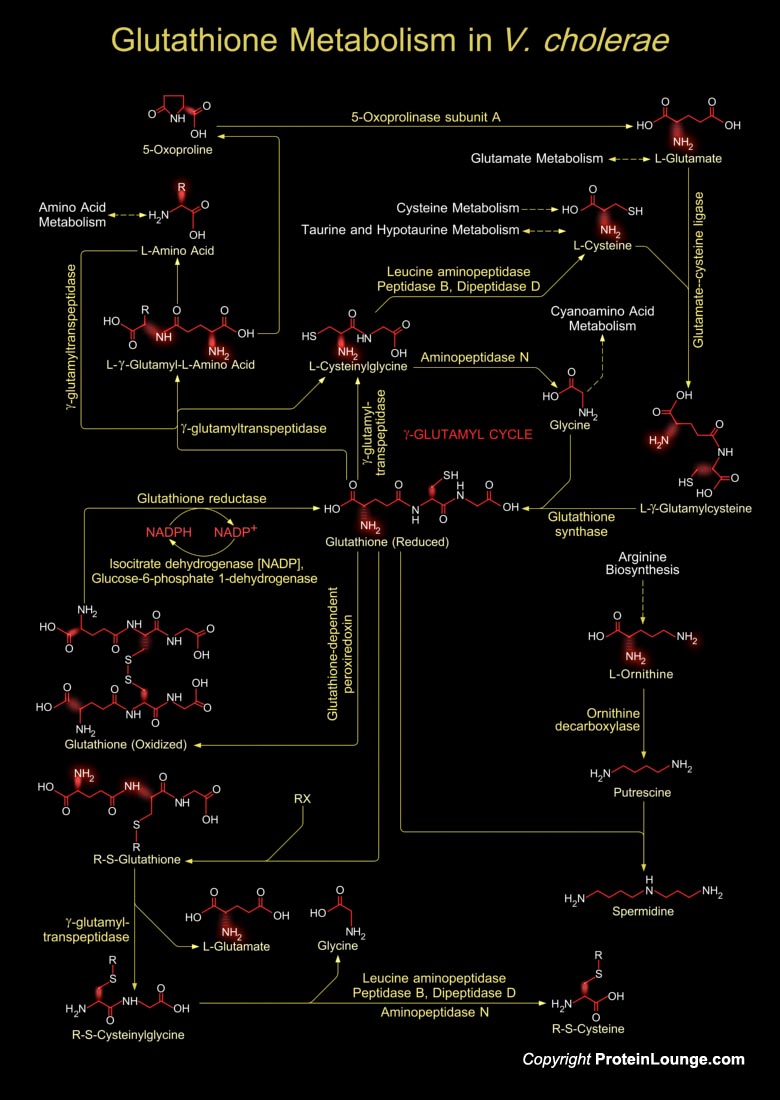
Vibrio cholerae is a facultative anaerobic, Gram-negative, crescent-shaped, motile rod like bacterium, and the causative infectious agent of the diarrheal disease, Cholera. It colonizes the mucosal surface of the human small intestine and secretes cholera toxin. The toxin stimulates secretion of water and electrolytes by the cells of the small intestine, leading to the severe watery diarrhoea that is characteristic of cholera. Unlike most bacterial agents of diarrhea, V. cholerae tends to survive in aquatic environments during periods between epidemics and can give rise to dramatic outbreaks of disease(Ref.1).Glutathione in V. cholerae is a tripeptide, composed of glutamate, cysteine and glycine, and has numerous important functions within the bacterial cell. This[..]
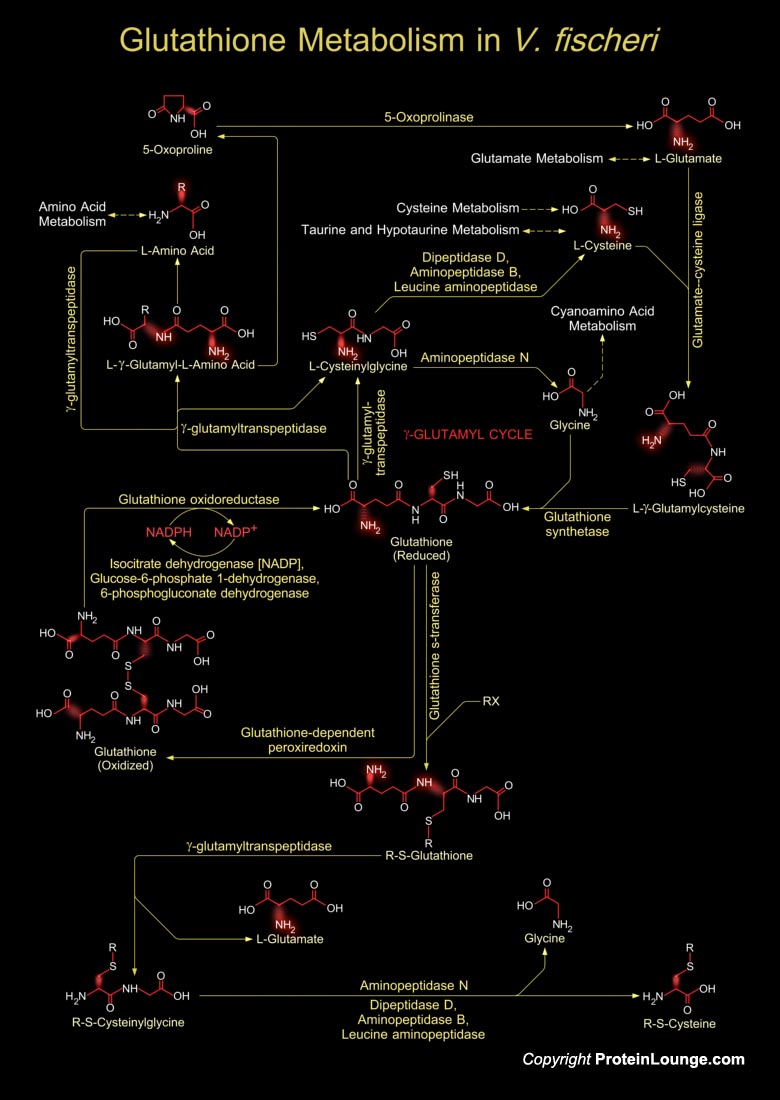
Vibrio fischeri is a Gram-negative heterotrophic bacterium, belonging to the Vibrionaceae, a large family within the Gamma-proteobacteria, consisting of many species that are characterized by both cooperative and pathogenic interactions with animal tissue. V. fischeri has a worldwide distribution, principally in temperate and subtropical waters, where it occupies a variety of niches. In addition to being a light-organ symbiont of several species of squids and fishes, this bacterium occurs as a member of the enteric consortia of many marine animals, as a pathogen of certain invertebrates and as a 'free-living' saprophyte growing on dissolved and particulate organic matter. In locations where it forms light-organ symbiosis with animals, free-living V. fischeri[..]
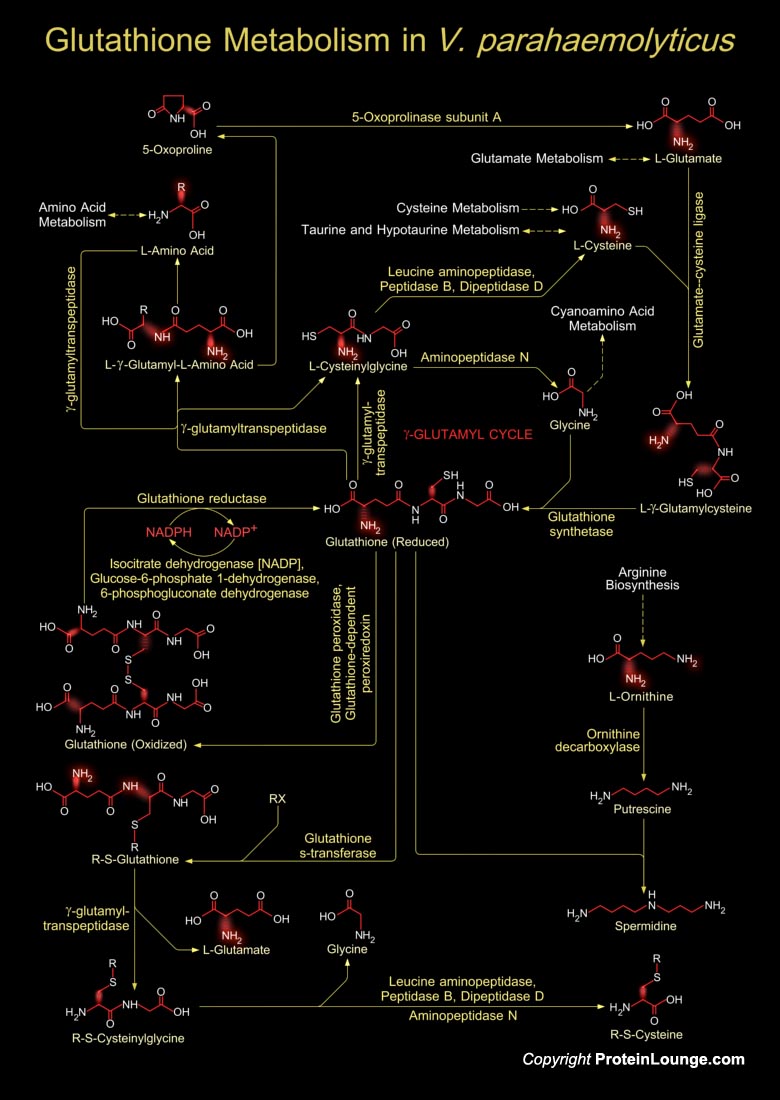
Vibrio parahaemolyticus, a Gram-negative marine bacterium, is a worldwide cause of food-borne gastroenteritis. The organism is phylogenetically close to V. cholerae, the causative agent of cholera. This universal marine pathogen is used as a bacterial model to clarify the various physiological phenomena of its native and host environments (Ref.1 & 2).Glutathione in V. cholerae is a tripeptide composed of Glutamate, Cysteine and Glycine, and has numerous important functions within the bacterial cell. This tripeptide is specifically a thiol compound, present in the highest concentration in all types of cells. During Glutathione metabolism, the laevorotatory amino acids present in the bacterium are used as precursors for ultimately synthesizing Glutathione, the[..]
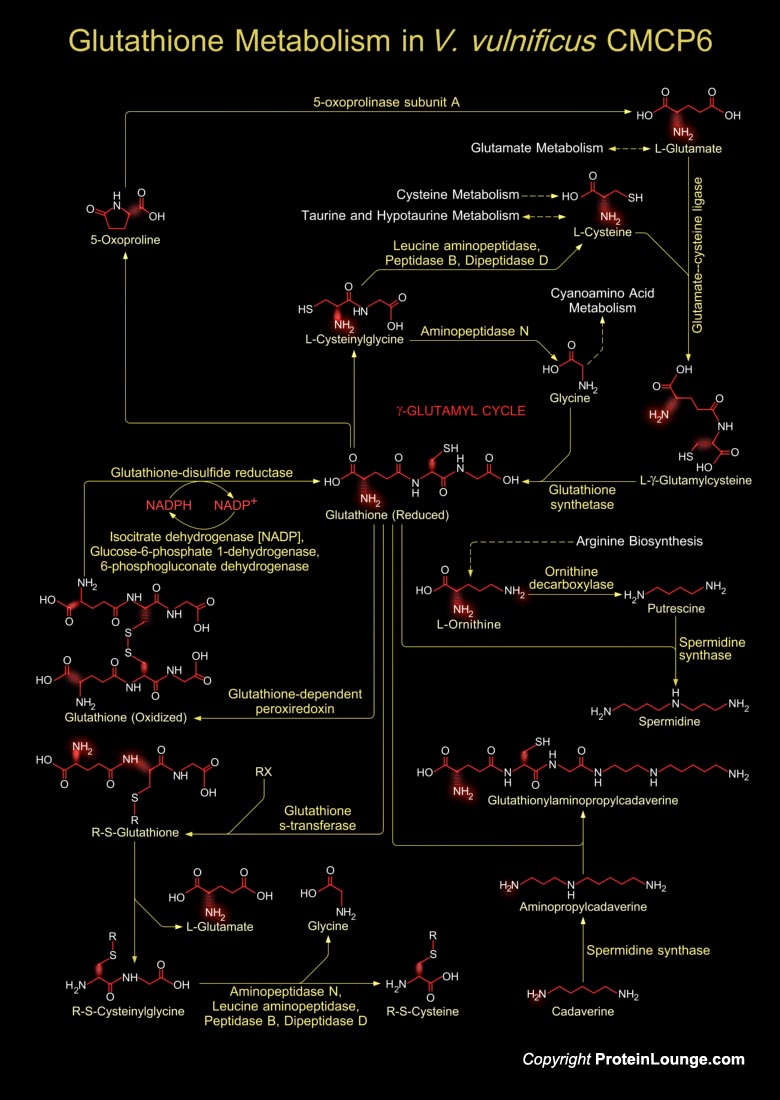
Vibrio vulnificus is an etiologic agent for severe human infection acquired through wounds or contaminated seafood. This is a lactose-fermenting, halophilic, Gram-negative, opportunistic pathogen, is found in estuarine environments and is associated with various marine species such as plankton, shellfish (Oysters, Clams, and Crabs), and finfish (Ref.1). V. vulnificus belong to the Gamma-group of Proteobacteria, and it shares morphological and biochemical characteristics with other human Vibrio pathogens, including Vibrio cholerae and Vibrio parahaemolyticus. It is divided into three biotypes according to its different biochemical and biological properties. V. vulnificus CMCP6 is a new strain of this bacterium, classified as Biotype-3 and is the sole cause of the[..]

