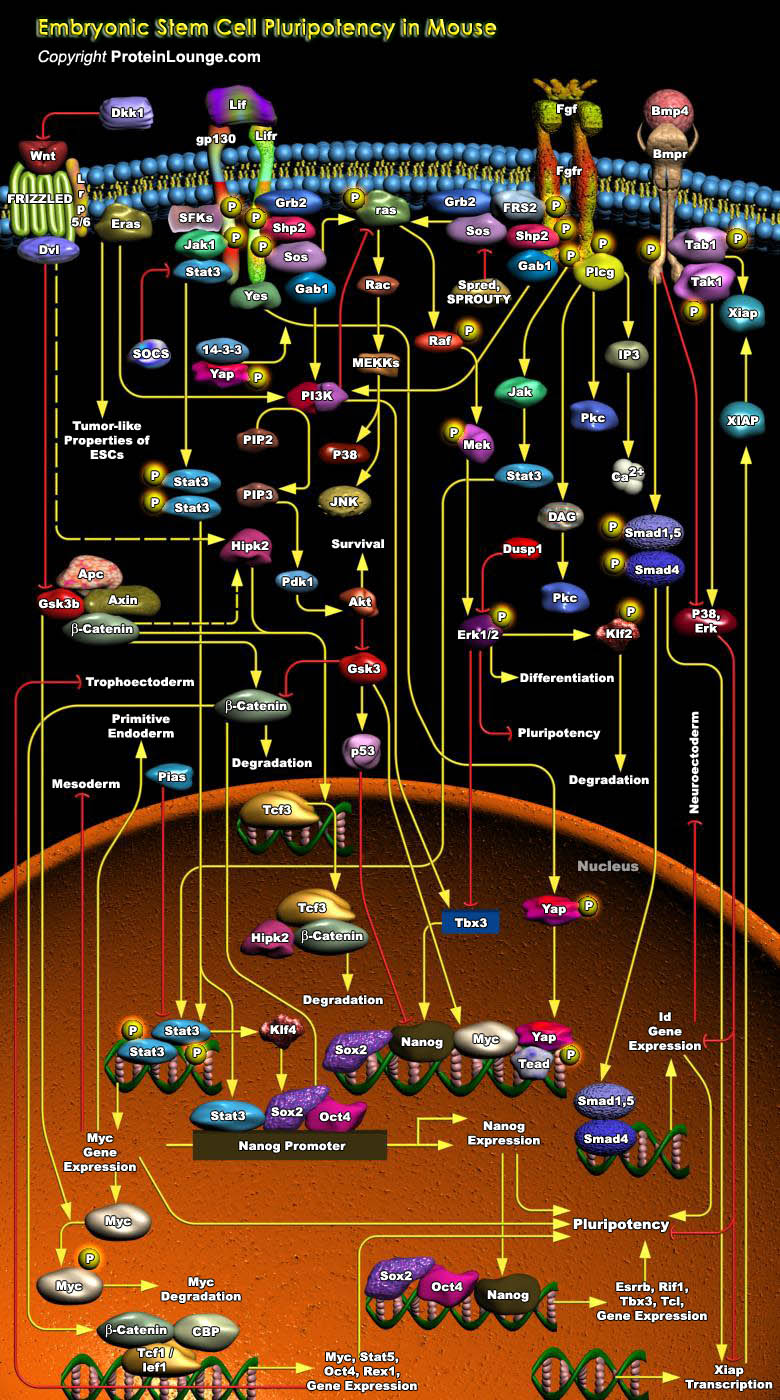
ESCs (Embryonic Stem Cells) are a population of Pluripotent, Self-renewing cells which can proliferate indefinitely and contribute to the formation of basically all cell types in vitro and in vivo. The study of mammalian ESCs, especially Mouse ESCs, has provided valuable insights into early embryogenesis in mammals. Mouse ES cells are derived mainly from the ICM (Inner Cell Mass) of the Mouse Blastocyst embryos and retain this developmental identity even after prolonged culture in vitro. Both cell Extrinsic and Intrinsic factors regulate Mouse ESC Self-renewal and Pluripotency. Cell extrinsic factors include LIF (Leukaemia Inhibitory Factor) and BMP (Bone Morphogenic Protein), which signal through STAT (Signal Transducer and Activator of Transcription) and SMAD (Sma[..]
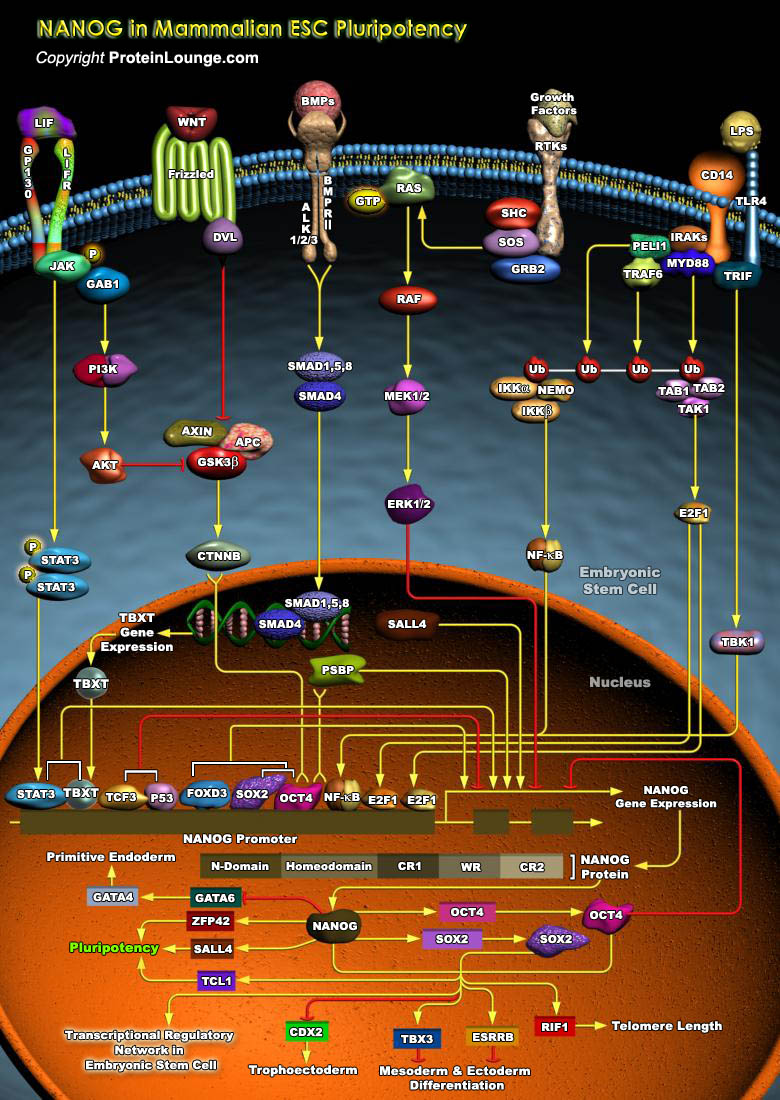
ESCs (Embryonic stem cells) are Pluripotent cells derived from the ICM (Inner Cell Mass) of Blastocyst-stage embryos. These cells have two distinctive properties: an unlimited capacity for Self-renewal and Pluripotency. The capability for Self-renewal and the Pluripotency of ESCs seem to be under the control of multiple transcriptional factors, most common among them being Nanog (Nanog homeobox), Oct4 (Octamer Binding Transcription Factor-4) and SOX2 (SRY (Sex Determining Region-Y) Box-2). Functions of these transcription factors depend on the stage of development of a Pluripotent cell, indicating that these factors function in combination with other processes. The activity of these transcription factors also depends on the accessibility[..]
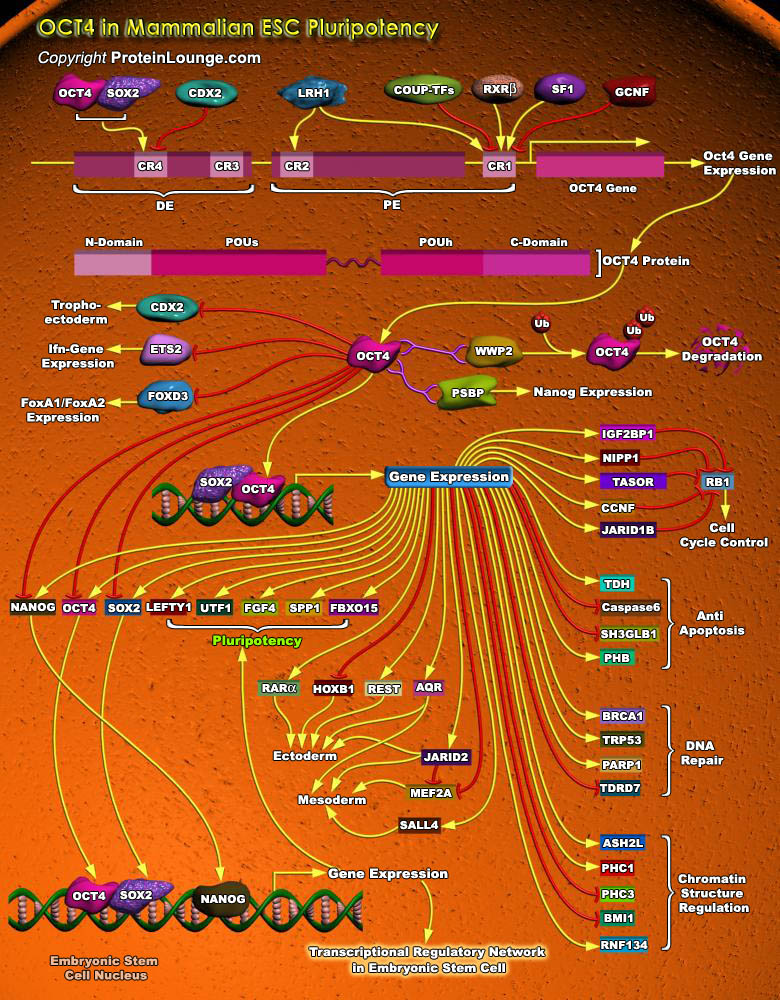
Stem cells are undifferentiated cells capable of producing virtually all cell types in our body. They are characterized by the ability to Self-renew and maintain Pluripotency. For proper developmental outcome, ESCs (Embryonic Stem Cells) must tightly regulate their differentiation status. Hundreds of genes have been identified, including several transcription factors, which have expression patterns tightly correlated with ESC differentiation. Very low number of transcription factors is responsible for regulating the development of an entire organism. These transcription factors form multiprotein complexes on DNA, thereby orchestrating the correct temporal–spatial expression of developmental genes. The process leads to the establishment of functional[..]
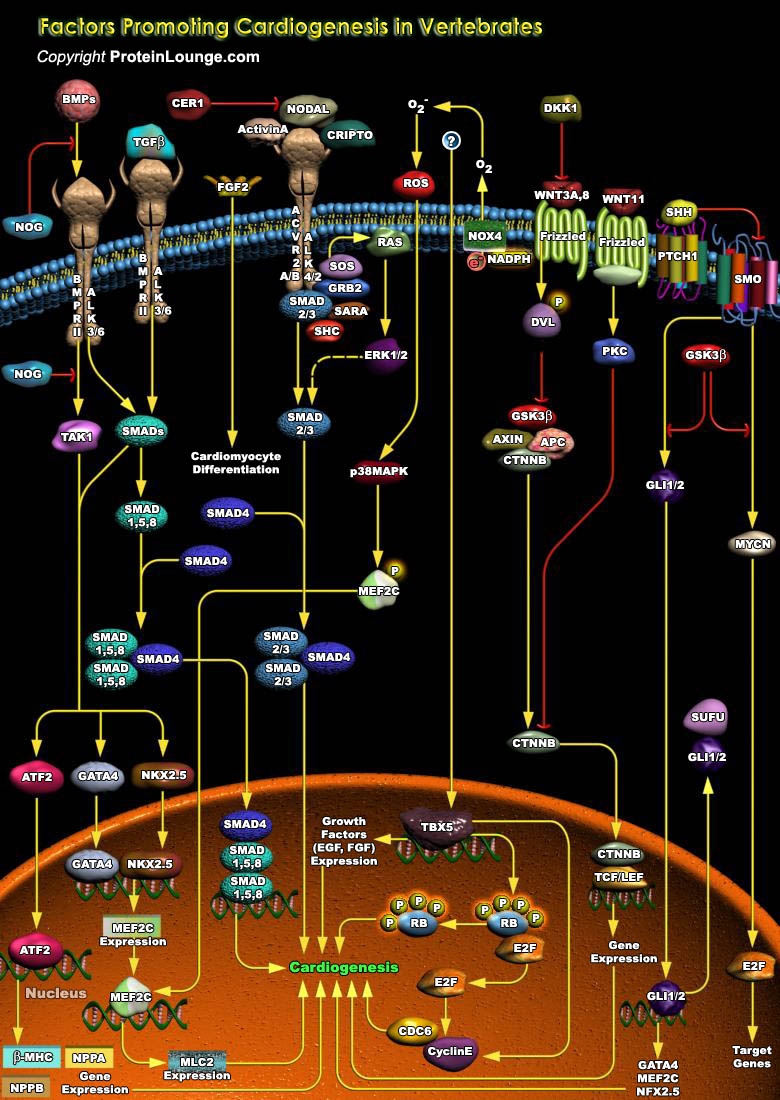
Cardiogenesis is the formation of new heart tissue from embryonic, postnatal, or adult multipotent cardiovascular progenitor cells. Cardiogenesis in vertebrate is a complex process, where various genetic and epigenetic factors play crucial role in driving the interaction between different structures and diverse cell types. Cardiomyocytes are the main cell type found in the heart that are responsible for the contraction of the chambers and efficient blood flow throughout the body (Ref.1 and 2). Multiple transcription factors and extracellular growth factors specify the Cardiac lineage in mesodermal progenitor cells. The earliest expressed transcription factors that initiate Cardiac fate are the homeobox transcription factor NKX2.5 (NK2 Transcription Factor Related[..]
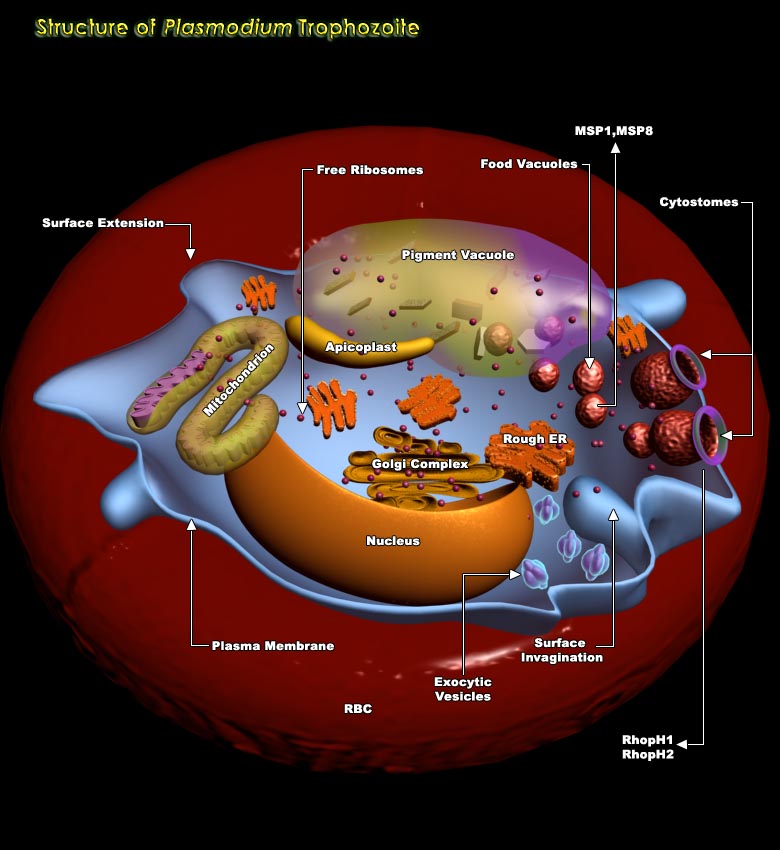
In the life cycle of Plasmodium spp, Erythrocytic stage is very important involving four stages viz. Merozoite stage, ring stage, Trophozoite stage and Schizont stage. On being released from the hepatocytes, the Merozoites enter the bloodstream prior to infecting red blood cells. They use the Apicomplexan invasion organelles to recognize and enter the host erythrocyte. The parasite first binds to the erythrocyte in a random orientation. It then reorients such that the apical complex is in proximity to the erythrocyte membrane. A tight junction is formed between the parasite and erythrocyte. As it enters the red blood cell, the parasite forms a parasitophorous vesicle, to allow for its development inside the erythrocyte. After invading the erythrocyte, the parasite[..]
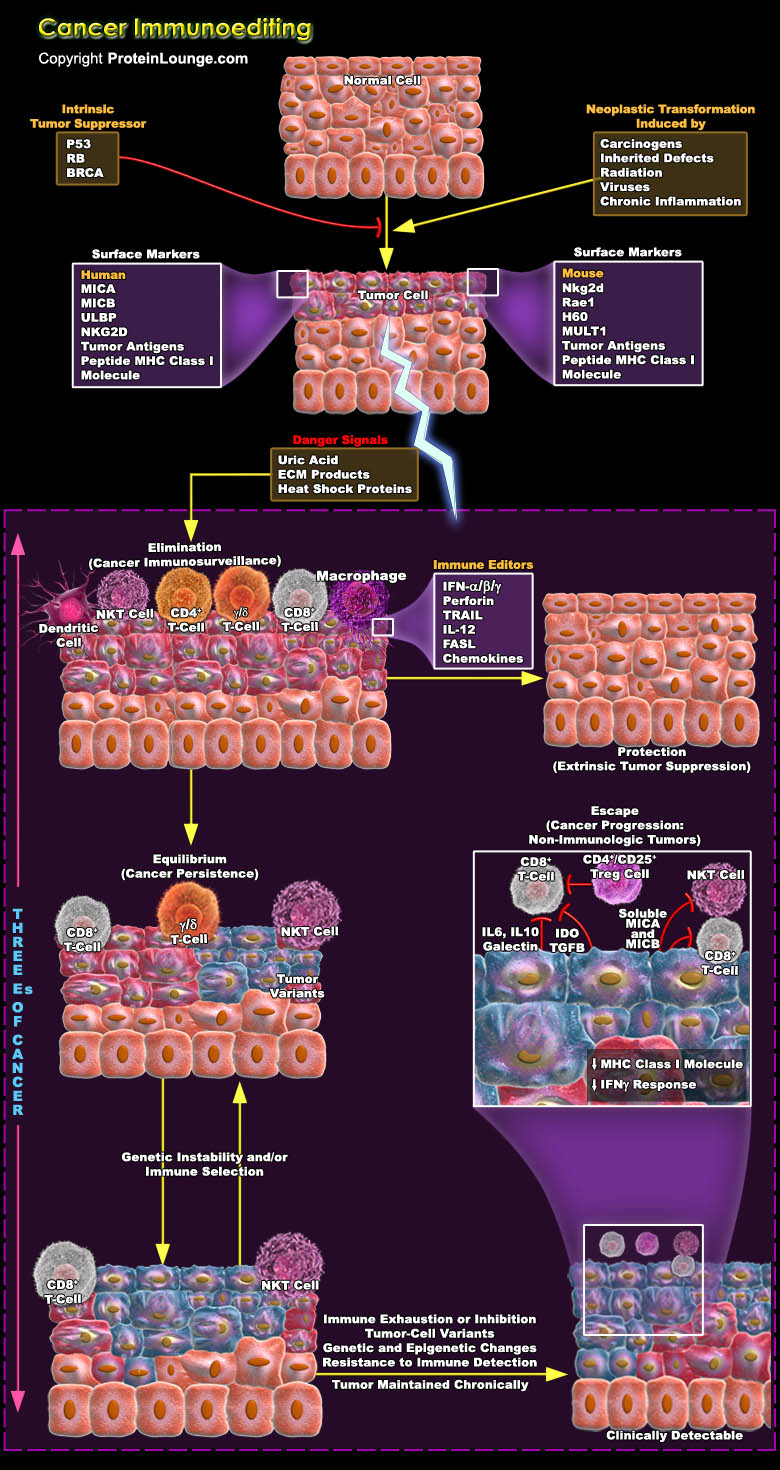
As research into tumour immunology continues at an incredible pace, a considerable amount of work is aimed at exploring the mechanisms that underlie the immunological recognition and elimination of cancer and the downstream consequences of these processes. The capacity of the immune system for recognition is not limited solely to the classic models of self versus pathogen or self versus non-self but encompasses the more-subtle differences that exist between self and transformed self. This conclusion provides the argument for reconsidering the largely discarded hypothesis of cancer immunosurveillance. Immune system attempts to constrain tumour growth, but sometimes tumour cells might escape or attenuate this immune pressure, similar to the way in which these cells[..]
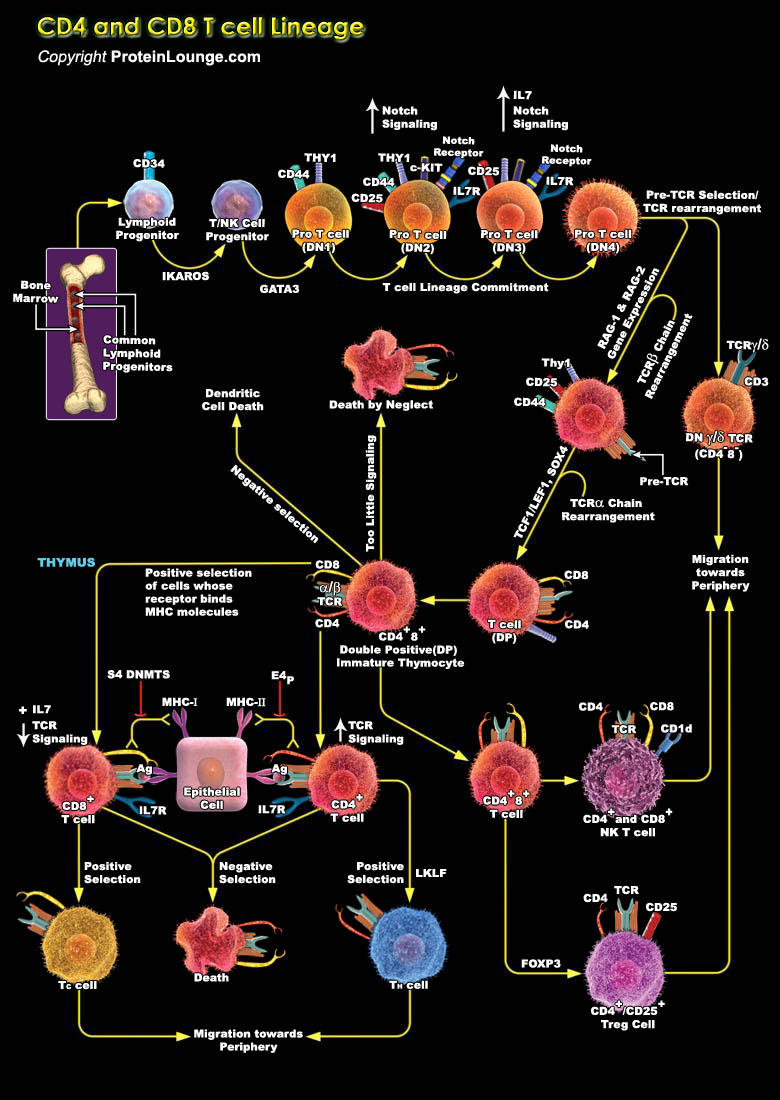
Cell-fate decisions are controlled typically by conserved receptors that interact with co-evolved ligands. Therefore, the lineage-specific differentiation of immature CD4+CD8+ T cells into CD4+ or CD8+ mature T cells is unusual in that it is regulated by clonally expressed, somatically generated T-cell receptors (TCRs) of unpredictable fine specificity. Each mature T cell generally retains expression of the co-receptor molecule (CD4 or CD8) that has an MHC-binding property that matches that of its TCR. Two models were proposed initially to explain this remarkable outcome--'instruction' of lineage choice by initial signalling events or 'selection' after a stochastic fate decision that limits further development to cells with coordinated TCR and co-receptor[..]
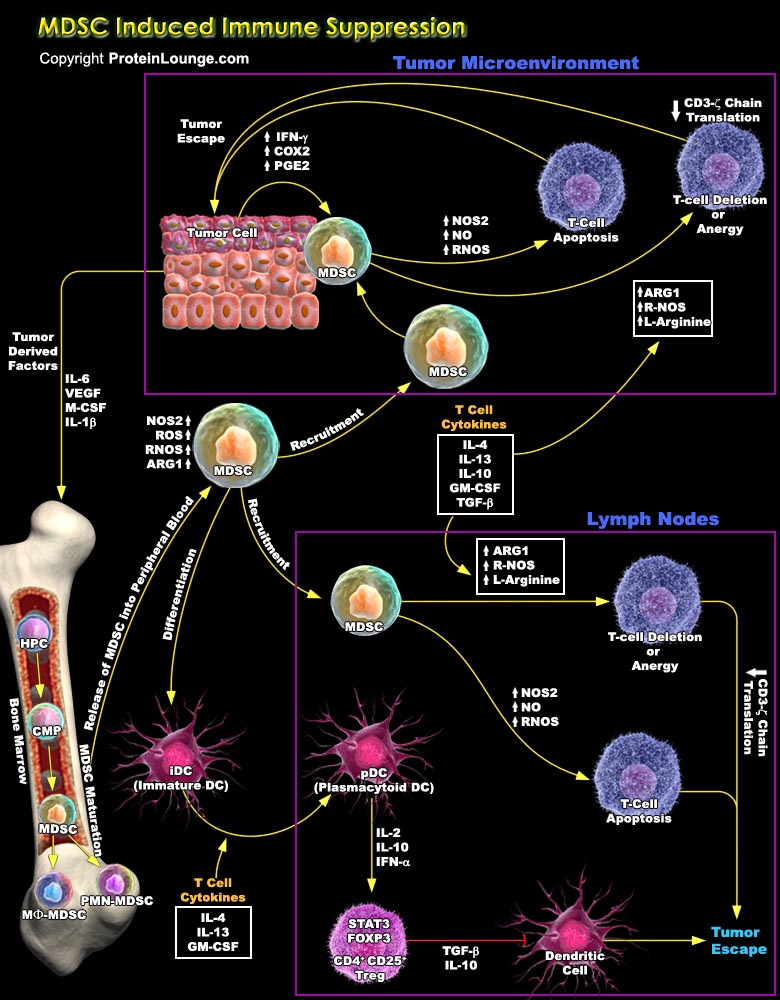
MDSCs (Myeloid-Derived Suppressor Cells) are recently been recognized as critical mediators of tumor progression in numerous solid tumours through their inhibition of tumor-specific immune responses. These cells are increased in numerous pathologic conditions, including infections, inflammatory diseases, graft-versus-host disease, traumatic stress, and neoplastic diseases. MDSCs inhibit not only activation of T cells by anti-CD3 and super antigen, but also antigen-specific CD4+ and CD8+ T-cell responses. The mechanisms of MDSC immunosuppression are diverse, it includes up-regulation of ROS (Reactive Oxygen Species), NO (Nitric Oxide), and L-Arginine metabolism. It also facilitates tumour-induced immune suppression and tumour progression by inducing the[..]
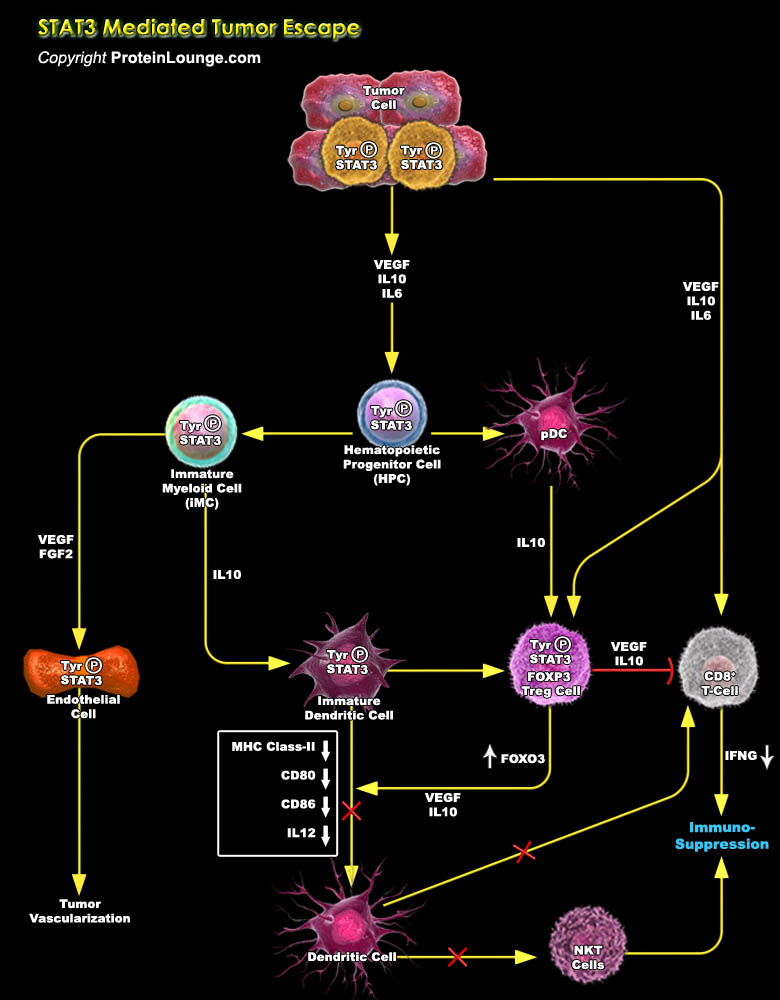
Immune cells in the tumour microenvironment not only fail to mount an effective anti-tumour immune response, but also interact intimately with the transformed cells to promote oncogenesis actively. STAT (Signal Transducer and Activator of Transcription) proteins act as a mediator of cytokine receptor signaling. This protein plays a role in transmitting the signals of growth factor receptors and is both cytoplasmic signaling molecules and nuclear transcription factors that activate diverse genes. In normal cells, STAT activation is transient and tightly regulated. While STAT3 (Signal Transducer and Activator of Transcription 3) acts as a point of convergence for numerous oncogenic signalling pathways, and constitutively remain active both in tumour[..]
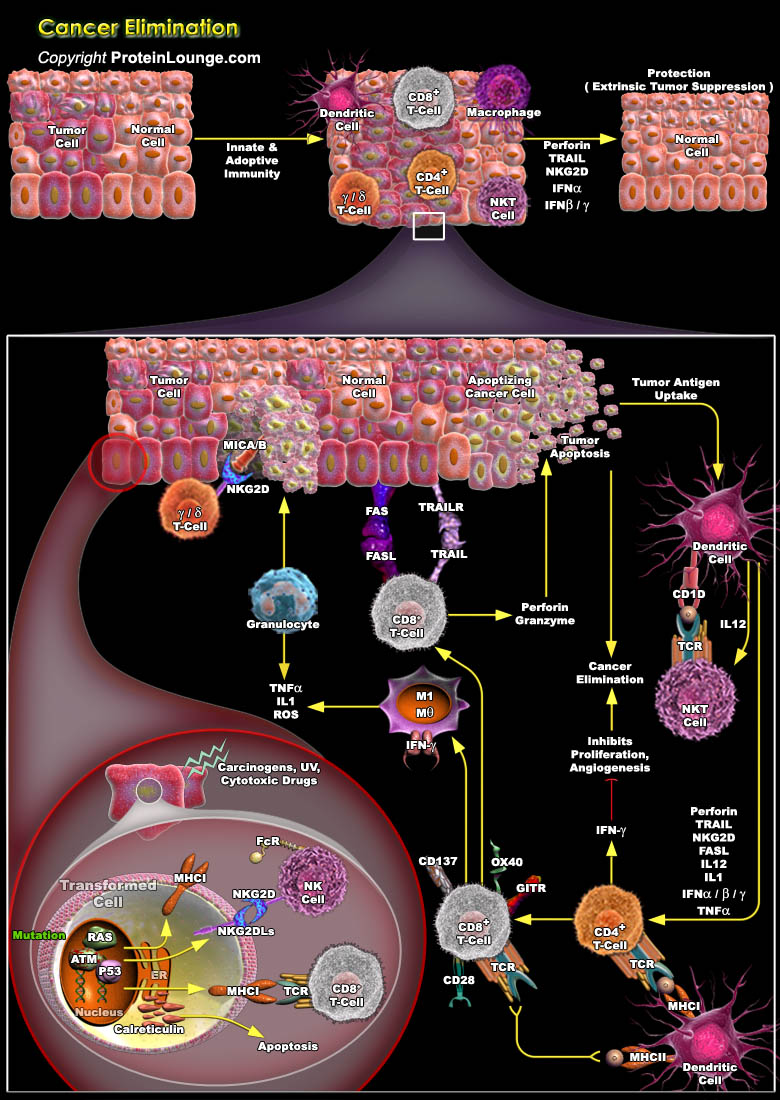
The immune system has three primary roles in the prevention of tumors. First, the immune system can protect the host from virus-induced tumors by eliminating or suppressing viral infections. Second, the timely elimination of pathogens and prompt resolution of inflammation can prevent the establishment of an inflammatory environment conducive to tumorigenesis. Third, the immune system can specifically identify and eliminate tumor cells on the basis of their expression of tumor-specific antigens or molecules induced by cellular stress. The evolution of the cancer immunoediting concept from the older and perhaps more controversial ‘cancer immunosurveillance’ hypothesis has helped interpret the predictive and prognostic significance of immune infiltrates into[..]
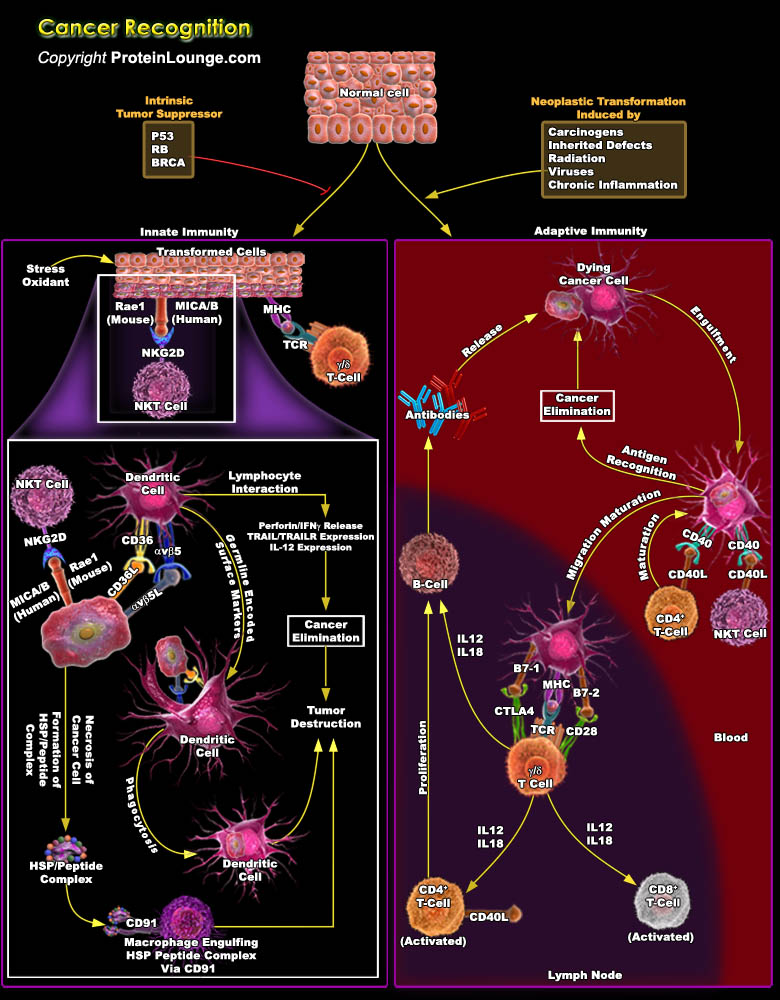
Cancer immunotherapy attempts to exploit the exquisite power and specificity of the immune system for the treatment of malignancy. Although cancer cells are less immunogenic than pathogens, the immune system is clearly capable of recognizing and eliminating tumour cells. However, tumors frequently interfere with the development and function of immune responses. Thus, the challenge for immunotherapy is to use advances in cellular and molecular immunology to develop strategies that effectively and safely augment antitumour responses. By contrast, the immune system has evolved strategies, largely in response to infections, to efficiently search for and specifically destroy diseased targets. Advances in cellular and molecular immunology have provides enormous insights in[..]
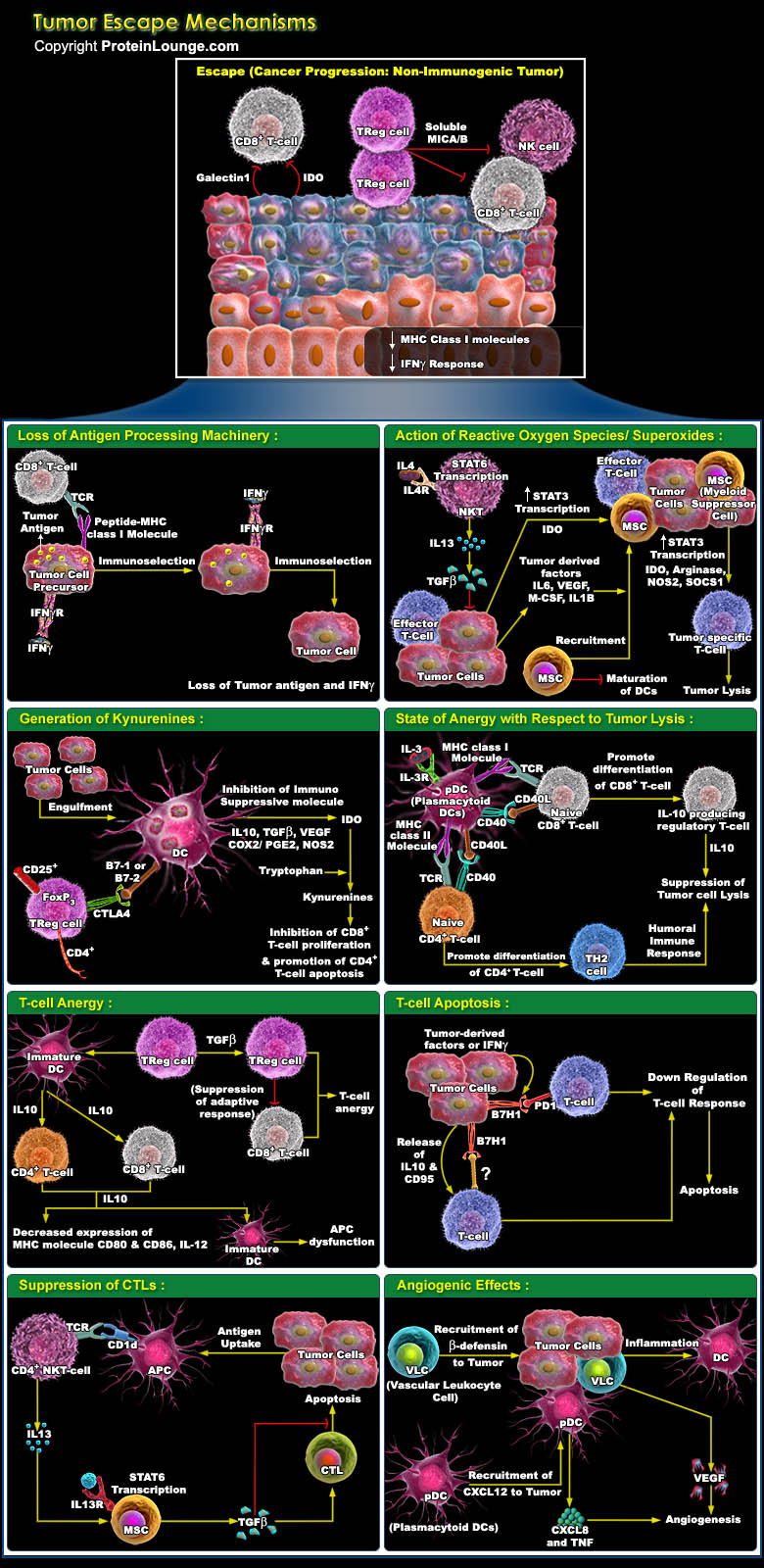
Tumour cells characteristically provides their own growth signals, and ignore growth-inhibitory signals, avoid cell death, replicate without limits, sustain angiogenesis, and invade tissues through basement membranes and capillary walls. Whereas cancer immunosurveillance predicts that the immune system can recognize precursors of cancer and, in most cases, destroy these precursors before they become clinically apparent. But sometimes tumour cells use to escape these innate and adaptive immune responses by immunoselection (that is, selection of non-immunogenic tumour-cell variants, a process that is also known as immunoediting) or by immunosubversion (that is, active suppression of the immune response) and heads towards a difficult situation which is refer as cancer.[..]

