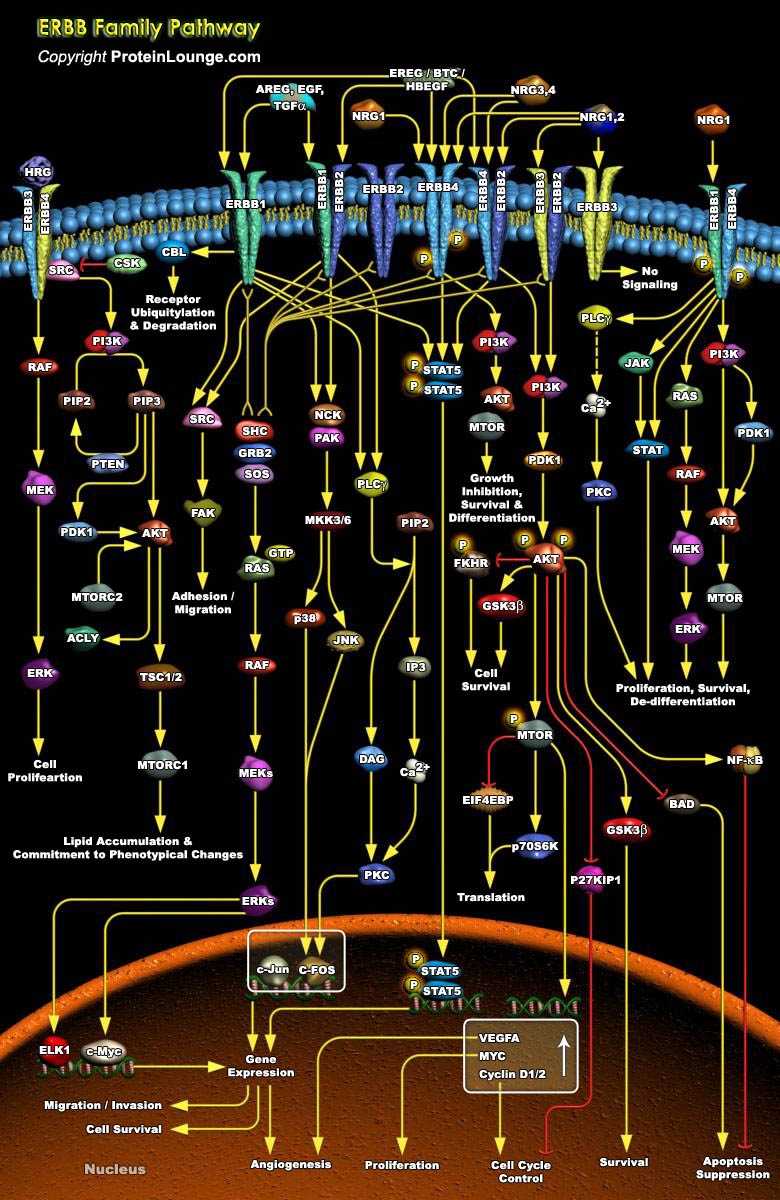
The ERBB (Erythroblastic Leukemia Viral Oncogene Homolog) or EGF (Epidermal Growth Factor) family of transmembrane RTKs (Receptor Tyrosine Kinases) plays an important role during the growth and development of a number of organs including the heart, the mammary gland, and the central nervous system. In addition, ERBB overexpression is associated with tumorigenesis of the breast, ovaries, brain, and prostate gland. The ERBB family includes four members, EGFR (EGF Receptor)/ERBB1/HER1 (Heregulin-1), ERBB2/HER2 (Heregulin-2), ERBB3/HER3 (Heregulin-3), and ERBB4/HER4 (Heregulin-4).Two of the family members, ERBB1 and ERBB2, are involved in the development of many types of human cancer. All ERBBs have in common an extracellular ligand-binding domain, a single[..]
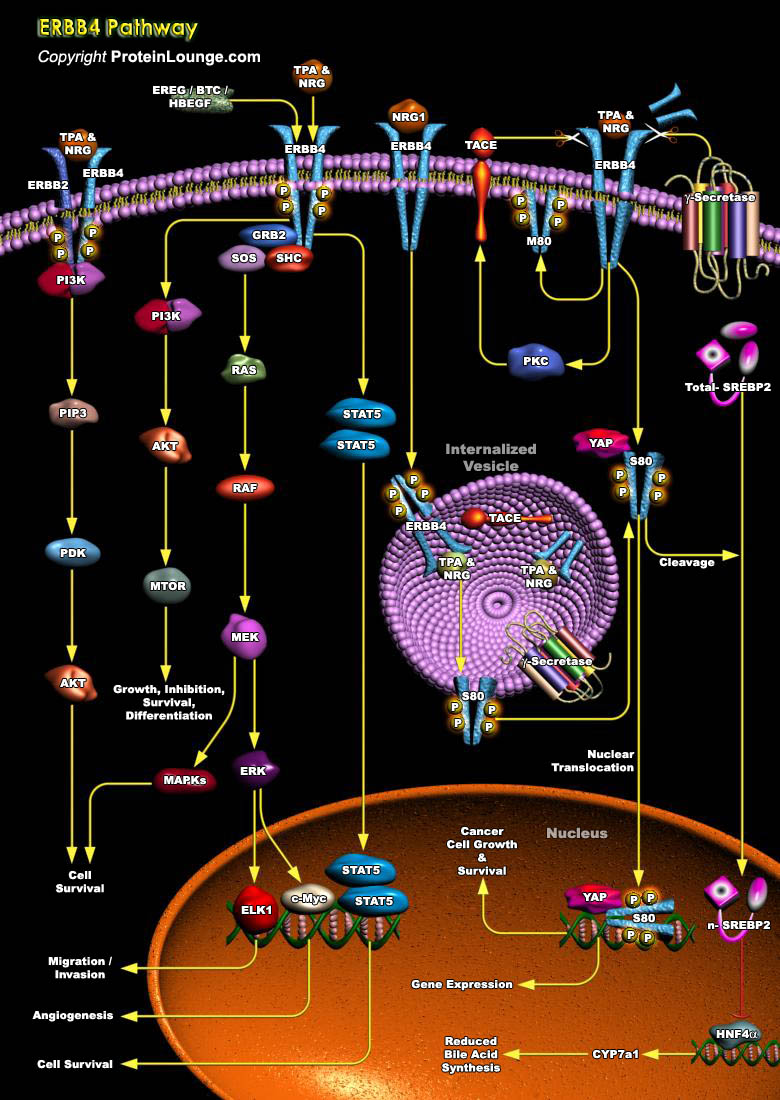
ERBB4 (Erythroblastic Leukemia Viral Oncogene Homolog-4) is a 180-kDa transmembrane RTK (Receptor Tyrosine Kinase) that regulates cell proliferation and differentiation. The ERBB4 is a member of the EGFR (epidermal growth factor receptor) subfamily of transmembrane RTKs. ERBB4 is expressed in several tissues, mainly heart, mammary gland and the central nervous system. ERBB4 and its ligands have important roles in normal cardiovascular and neural development, differentiation of the mammary gland, and in pathological conditions, such as heart diseases and cancer (Ref.1).ERBB4 can be activated by at least seven members of EGF-related peptide growth factors: betacellulin, epiregulin, HB-EGF (heparin-binding EGF-like growth factor) and the neuregulins (NRG-1, NRG-2, NRG-3,[..]
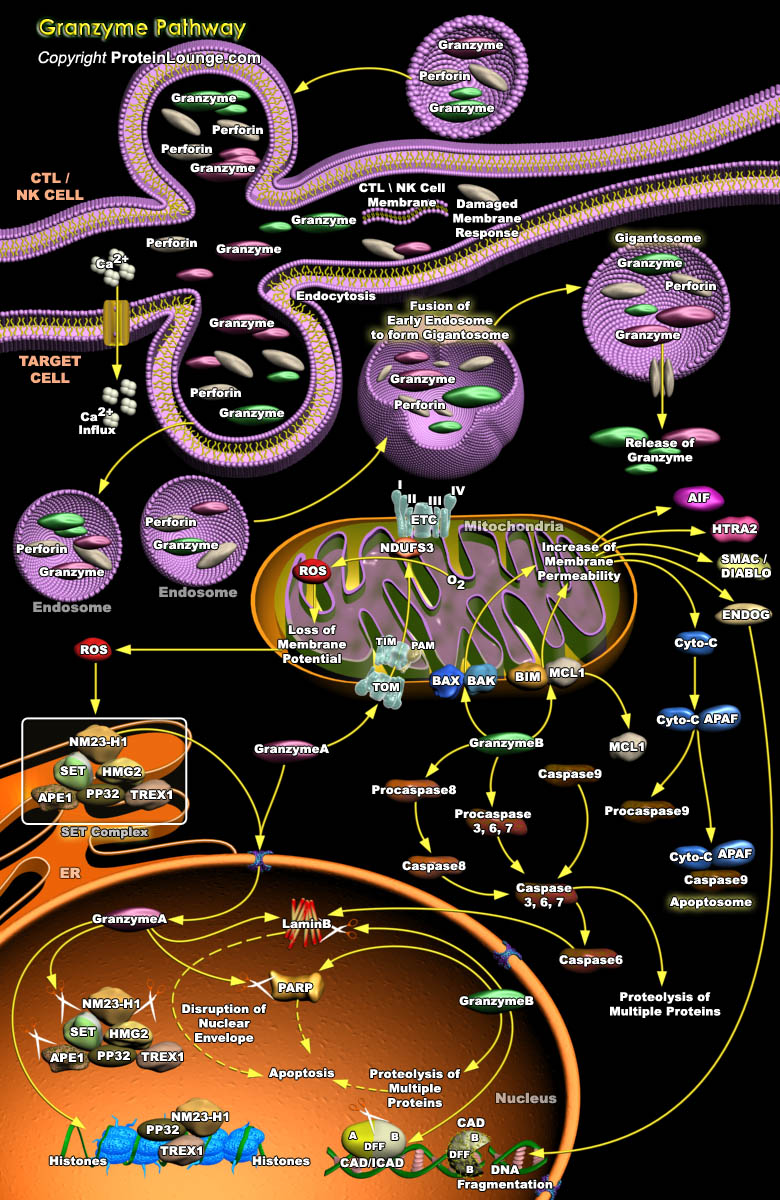
Killer lymphocytes are key players in the effector arm of the immune response that eliminate cells infected with intracellular pathogens and transformed tumor cells. Killer cells in both adaptive and innate immunity-T cells (CTLS) and natural killer (NK) cells, respectively, use the same basic mechanisms for destroying their targets, although they are triggered by distinct receptors and the expression of cytotoxic granules is constitutive in NK cells, but regulated in T cells. Release of the contents of cytotoxic granules into the immunological synapse formed between the killer cell and its target cell is important for immune elimination of viruses, intracellular bacteria, and tumors. Granzyme-A (GzmA), a tryptase and B (GzmB), serine protease, are the most abundant[..]
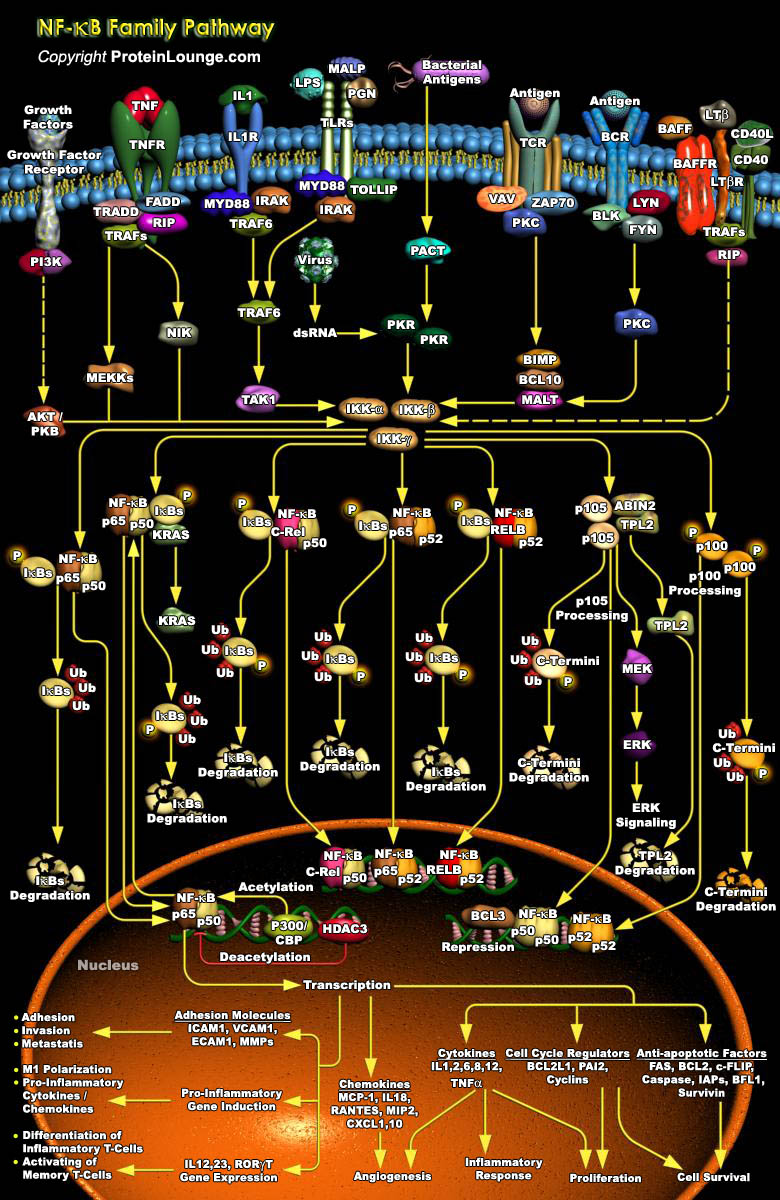
NF-KappaB (Nuclear Factor-KappaB) is a heterodimeric protein composed of different combinations of members of the Rel family of transcription factors. The Rel/ NF-KappaB family of transcription factors are involved mainly in stress-induced, immune, and inflammatory responses. In addition, these molecules play important roles during the development of certain hemopoietic cells, keratinocytes, and lymphoid organ structures. More recently, NF-KappaB family members have been implicated in neoplastic progression and the formation of neuronal synapses. NF-KappaB is also an important regulator in cell fate decisions, such as programmed cell death and proliferation control, and is critical in tumorigenesis (Ref.1).NF-KappaB is composed of homo- and heterodimers of five members[..]
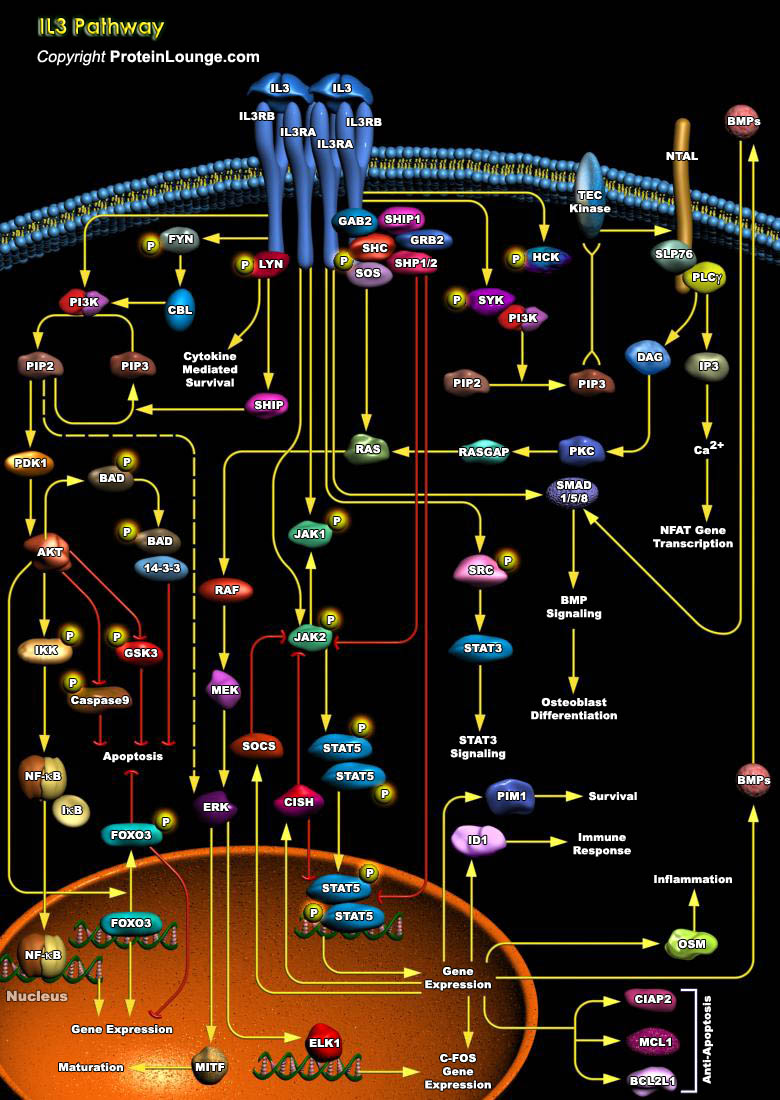
Interleukin 3 (IL3) is a T cell-derived glycoprotein involved in cell proliferation, survival and differentiation. It regulates haemopoiesis, the formation of blood cells in the body. IL3, also called multi-CSF (multi-lineage colony stimulating factor), is produced by T cells and mast cells, after activation with mitogens or antigens. It was first isolated from murine bone marrow cells and is also believed to be expressed in neurons and astrocytes of the hippocampus, suggesting an important role in central nervous system (Ref.1). It signals through interactions with cell surface receptors. IL3 receptor (IL3R) is composed of two polypeptide chains, a unique alpha subunit and a common Beta subunit IL3RB (the common beta chain), which is shared by[..]
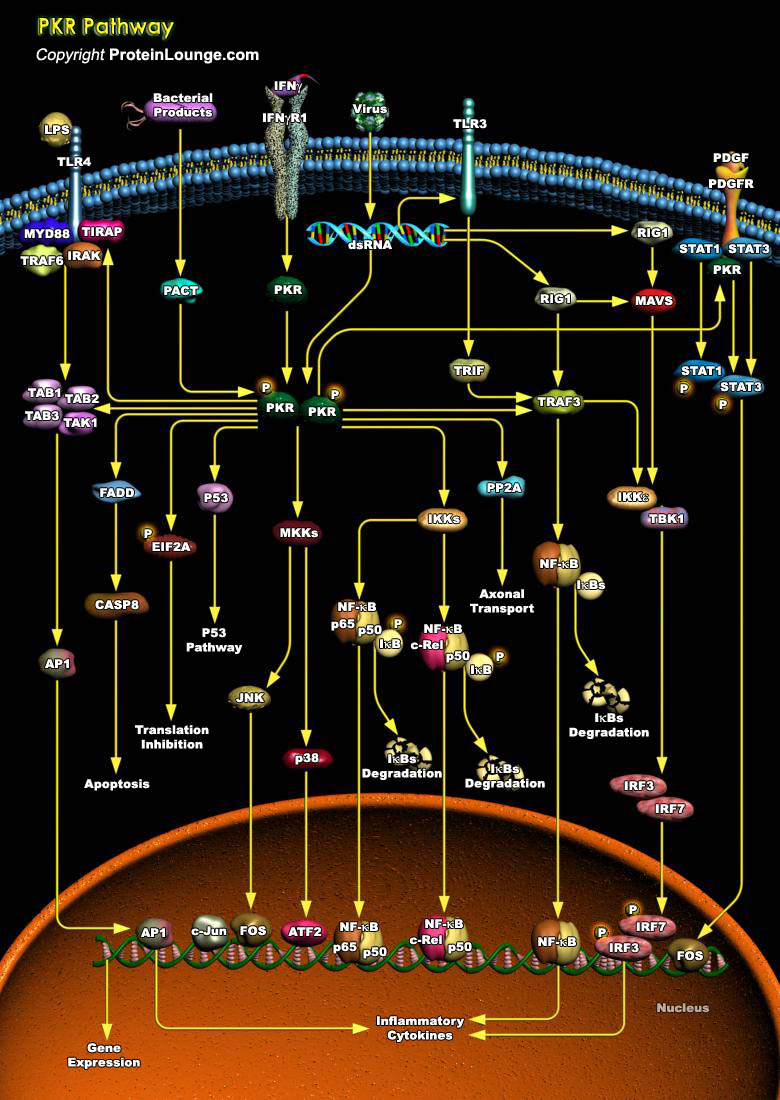
PKR (Protein kinase-R) is a ubiquitously expressed serine-threonine kinase that has been implicated as a signal integrator in translational and transcriptional control pathways. PKR mediate apoptosis induced by many different stimuli, such as LPS (Lipopolysaccharides), IFN-Gamma (Interferon-Gamma), cytokines, growth factor, viral infection, or serum starvation. PKR activity is regulated by external signals, which act on proteins, which interact with PKR. Among the proteins identified so far are p58, a cellular protein, K3L, the product of a gene of vaccinia virus, PACT (Protein Activator Of Interferon-Induced Protein Kinase) and p67, an interferon-induced glycoprotein (Ref.1). Human PKR is a 68 kDa with a 20 kDa N-terminal dsRBD[..]
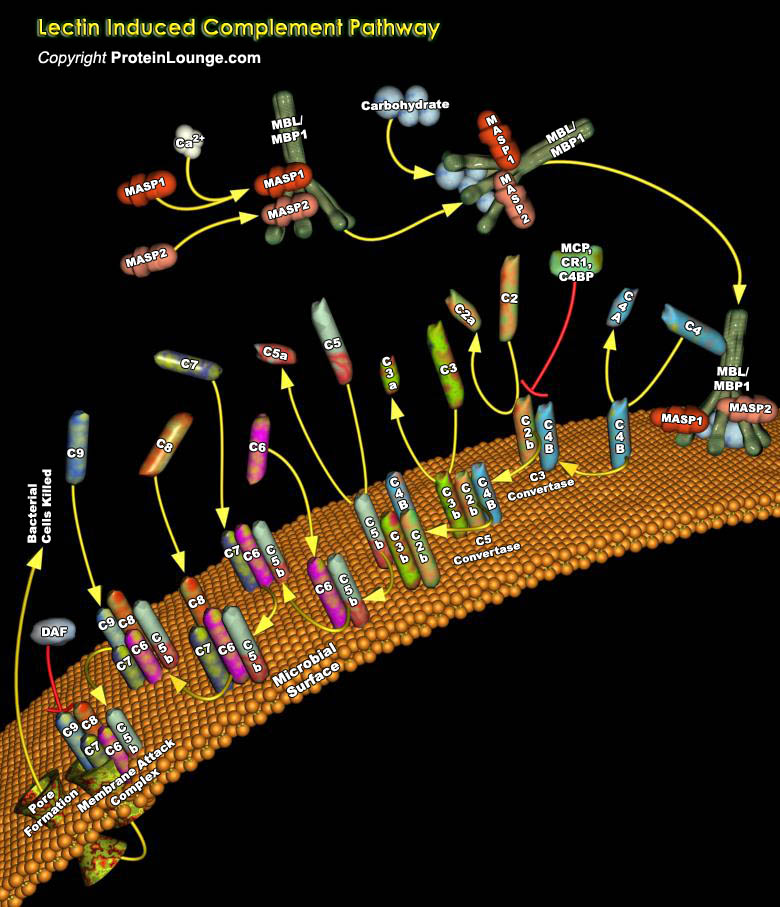
Complement is a complex system containing more than 30 various glycoproteins present in serum in the form of components, factors, or other regulators and/or on the surface of different cells in the form of receptors. It is a highly sophisticated host defence system designed to destroy pathogens. Once the complement system is activated, a chain of reactions involving proteolysis and assembly occurs, resulting in destruction of the membranes of pathogens. The cascade up to the cleavage of C3 (Complement component 3), which plays a central role in the complement system, is called the activation pathway. There are three categories of complement pathway; the classical pathway, alternative pathway and lectin pathway (Ref.1).The recently discovered lectin pathway (also called[..]
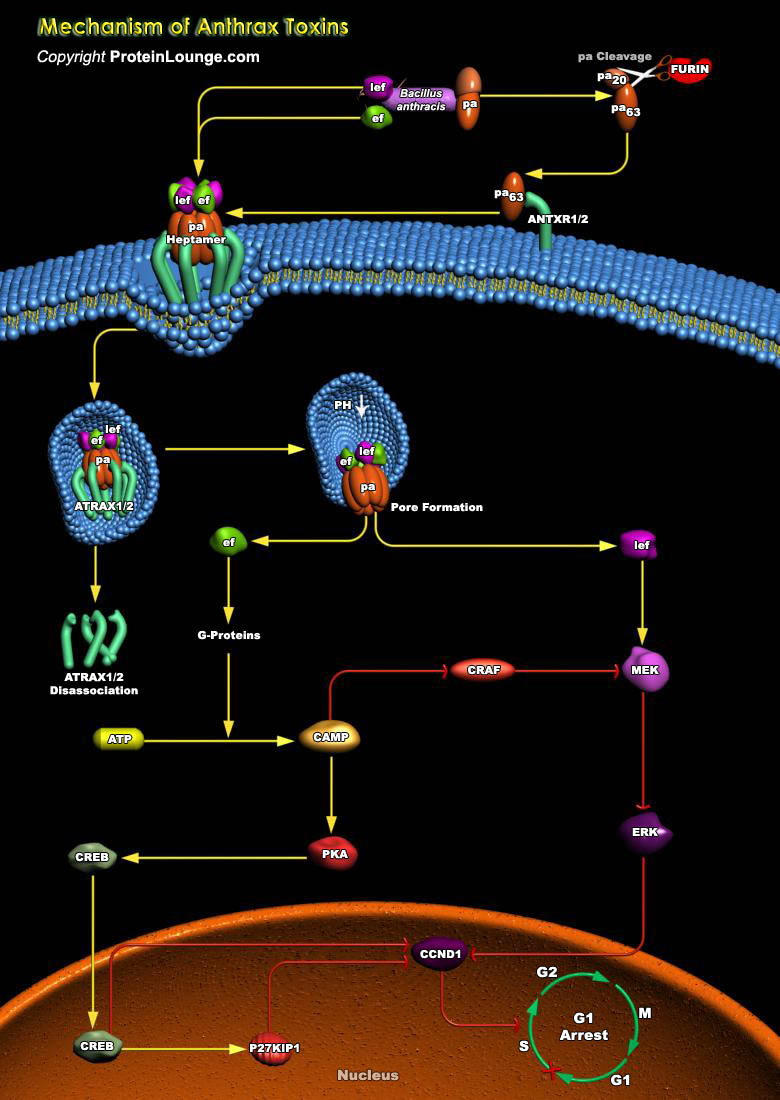
Anthrax is a severe, although rather rare, infectious disease that is caused by the Gram-positive, spore-forming bacterium Bacillus anthracis. The infectious form is the spore and the major virulence factors of the bacterium are its poly-Gamma-D-glutamic acid capsule and the tripartite anthrax toxin. The discovery of the anthrax toxin receptors in the early 2000s has allowed in-depth studies on the mechanisms of anthrax toxin cellular entry and translocation from the endocytic compartment to the cytoplasm. There are three forms of anthrax disease defined by the route of spore entry into the body: cutaneous, gastrointestinal, and inhalational anthrax. Early studies showed that spores are phagocytosed by resident macrophages and dendritic cells, which may serve as a[..]
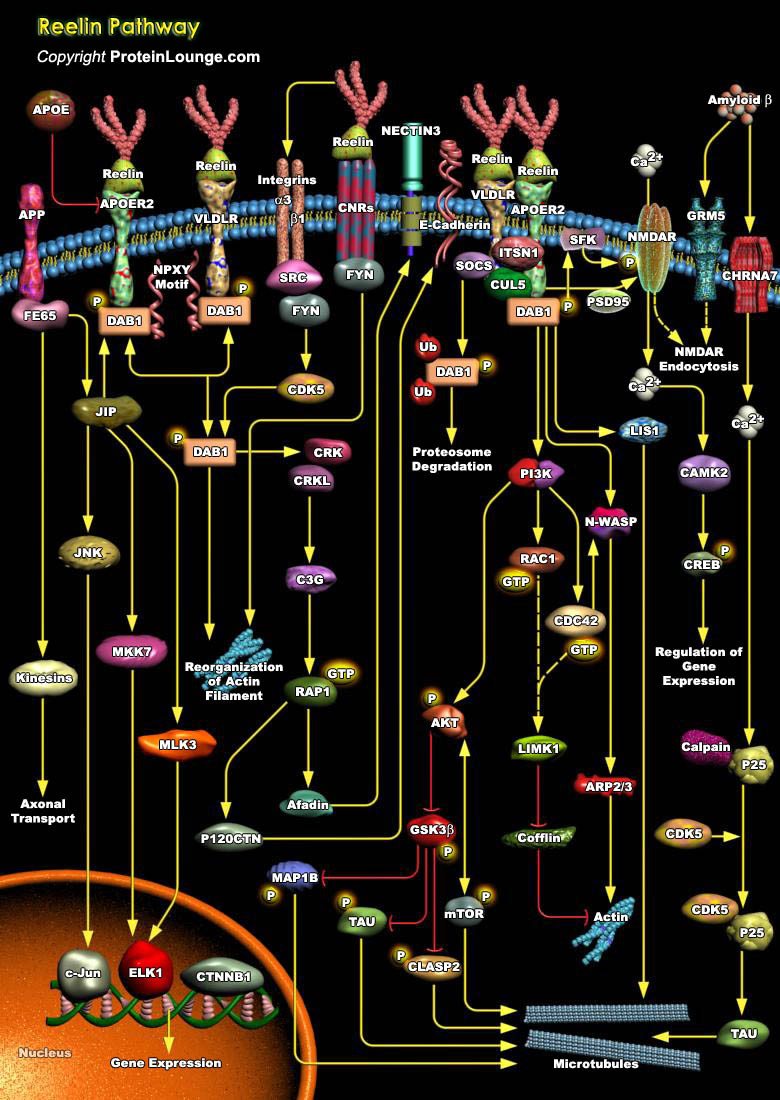
Reelin is a large extracellular glycoprotein involved in the development of architectonic patterns, particularly in the cerebral cortex and hippocampus, where primarily Cajal-Retzius cells synthesize it The Reelin signaling pathway controls neuronal migration and positioning in central nervous system (CNS) development, as well as synaptic plasticity and memory (Ref.1). Reelin is localized to chromosome 7 in human with serine protease activity containing 3461 amino acids. It contains a signal peptide followed by an N-terminal sequence and a hinge region upstream from eight Reelin repeats of 350–390 amino acids. Each Reelin consists of a signal peptide (S), an F-spondin-like domain (SL), eight consecutive Reelin repeats (R) each harboring an epidermal growth[..]
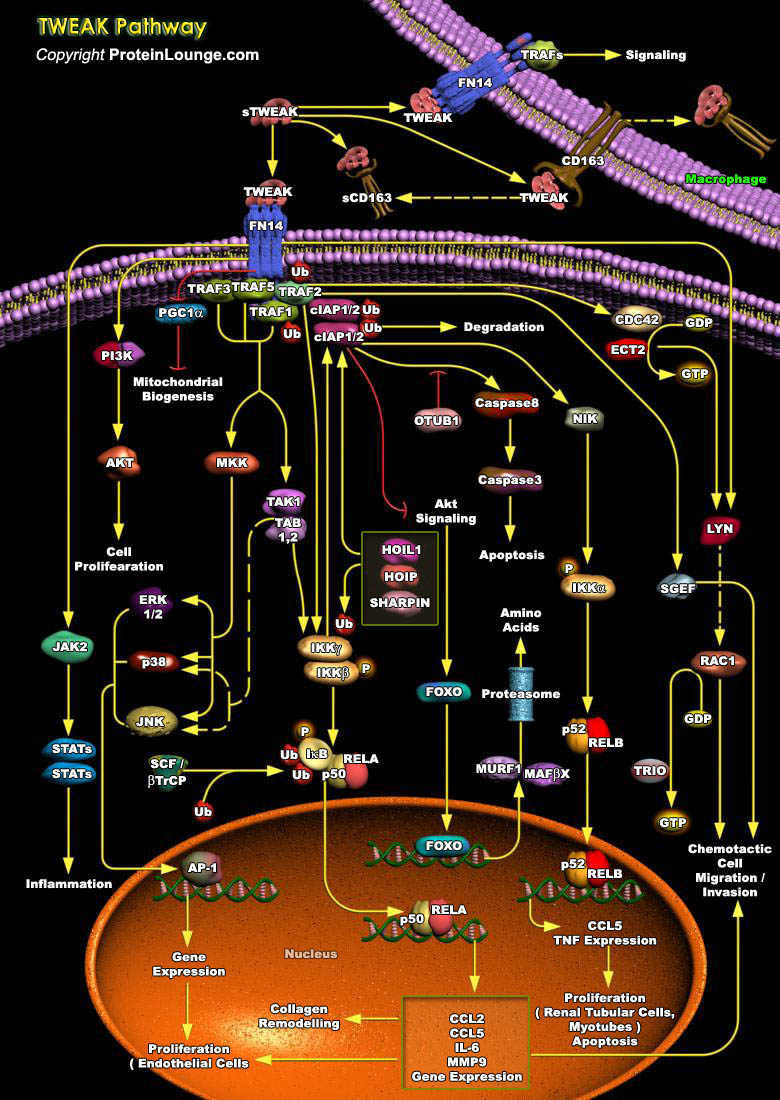
Tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK) is a member of the tumor necrosis factor (TNF) superfamily (TNFSF) that is induced in a variety of cell types in situations of tissue injury. It is expressed in various tissues including the intestine, pancreas, lung, brain, skeletal muscle, heart and vasculature. The 249 amino acid-comprising membrane-bound form of TWEAK consists extracellularly of the characteristic C-terminally located TNF homology domain (THD), which is separated from the transmembrane domain by a stalk region of about 55 amino acids. It can be cleaved by furin endoprotease into a soluble TWEAK (sTWEAK) (185 aa, 18 kDa) which then binds to the Fibroblast growth factor-inducible 14 (Fn14) receptor on the cell, the cognate receptor of[..]
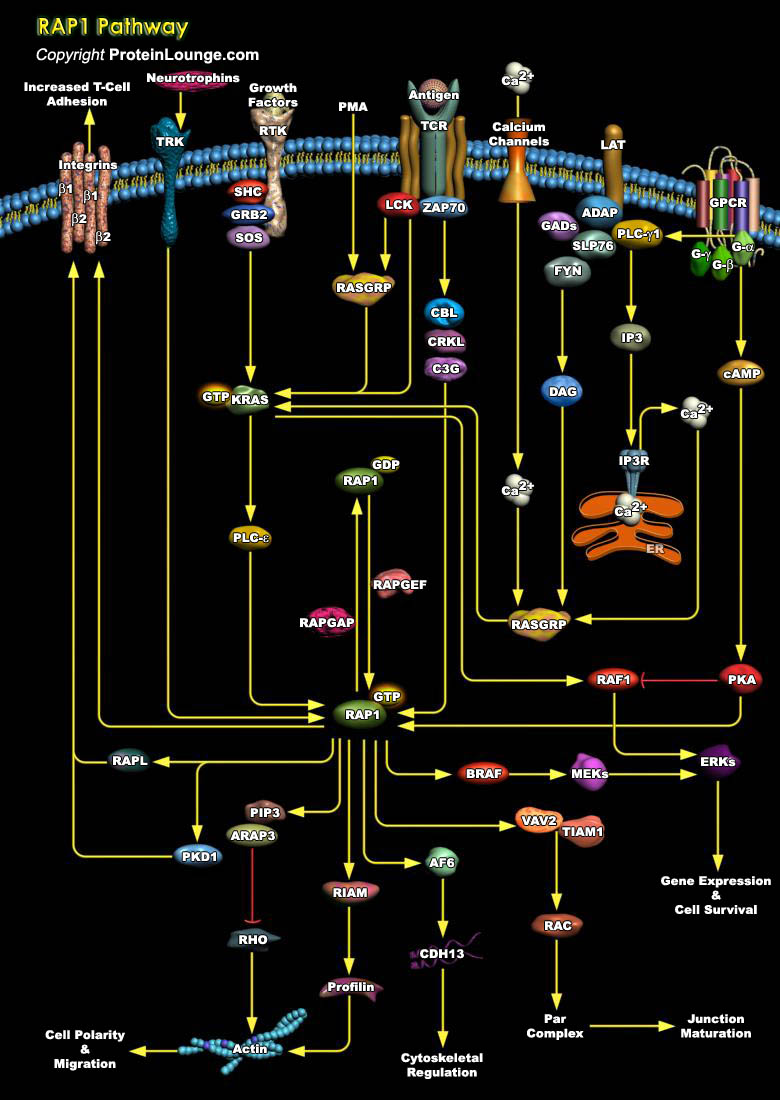
Rap1 (Krev-1/smg p21), a small-molecular-weight GTP-binding protein that belongs to the Ras-like superfamily of GTPases, is involved in signal transduction cascades. It is highly homologous to Ras but it is down regulated by its own set of GAPs (GTPase-Activating Proteins). Rap1 is implicated in the regulation of a variety of cellular processes including the control of platelet activation, T-cell anergy, B-Cell activation, and neuronal differentiation. Very recently, Rap was shown to be involved in the control of cell adhesion, in particular the regulation of integrin activation by inside-out signaling. In humans, four isoforms of Rap, Rap1A, Rap1B, Rap2A, and Rap2B, exist. Rap1A and Rap1B share more than 90% sequence identity. Rap1A/B is the closest[..]
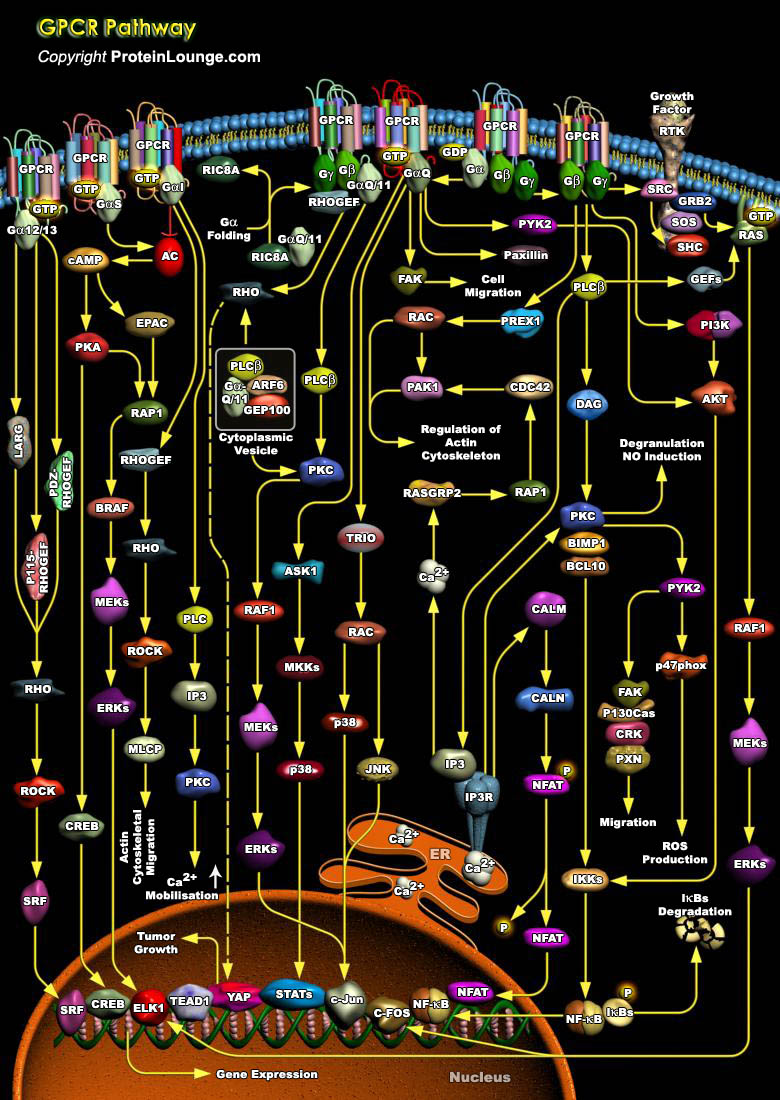
GPCRs (Guanine Nucleotide Binding–Protein Coupled Receptors) comprise large and diverse gene families in fungi, plants, and the animal kingdom. Also termed serpentine receptors, GPCRs are polytopic membrane proteins that share a common structure with seven transmembrane segments, but sequence similarity is minimal among the most distant GPCRs. Their principal function is to transmit information about the extracellular environment to the interior of the cell, and they do this by interacting with the G-proteins. GPCRs recognize a variety of ligands and stimuli including peptide and non-peptide hormones and neurotransmitters, chemokines, prostanoids and proteinases, biogenic amines, nucleosides, lipids, growth factors, odorant molecules and light. These receptors[..]

