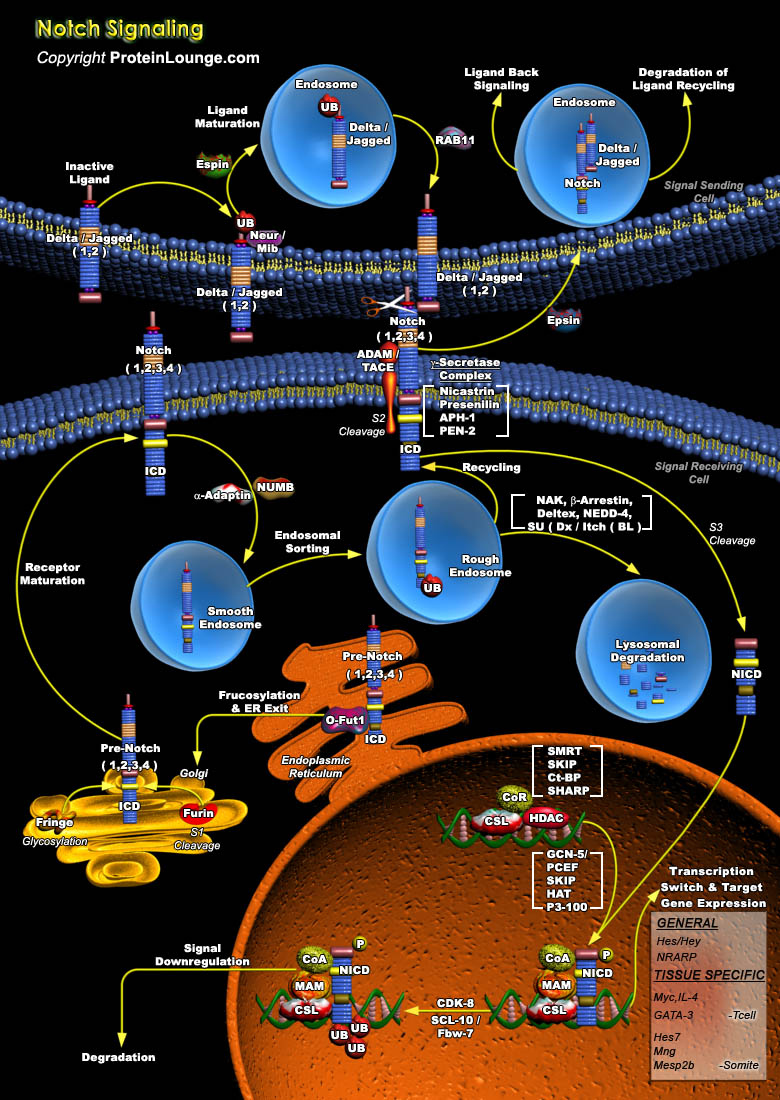
The Notch signaling pathway is a fundamental signaling system used by neighboring cells to communicate with each other in order to assume their proper developmental role. Notch proteins are cell surface transmembrane-spanning receptors which mediate critically important cellular functions through direct cell-cell contact. Interaction between Notch and its proposed ligands initiates a signaling cascade that governs cell fate decisions such as differentiation, proliferation, and apoptosis in numerous tissue types. The core elements of the Notch signaling system include the Notch receptor, DSL ligands (Delta and Serrate/Jagged in Drosophila and vertebrates, Lag2 in Caenorhabditis elegans) and CSL DNA-binding proteins (CBF1/RBPJ-kappa in vertebrates, Su(H) [Suppressor of[..]
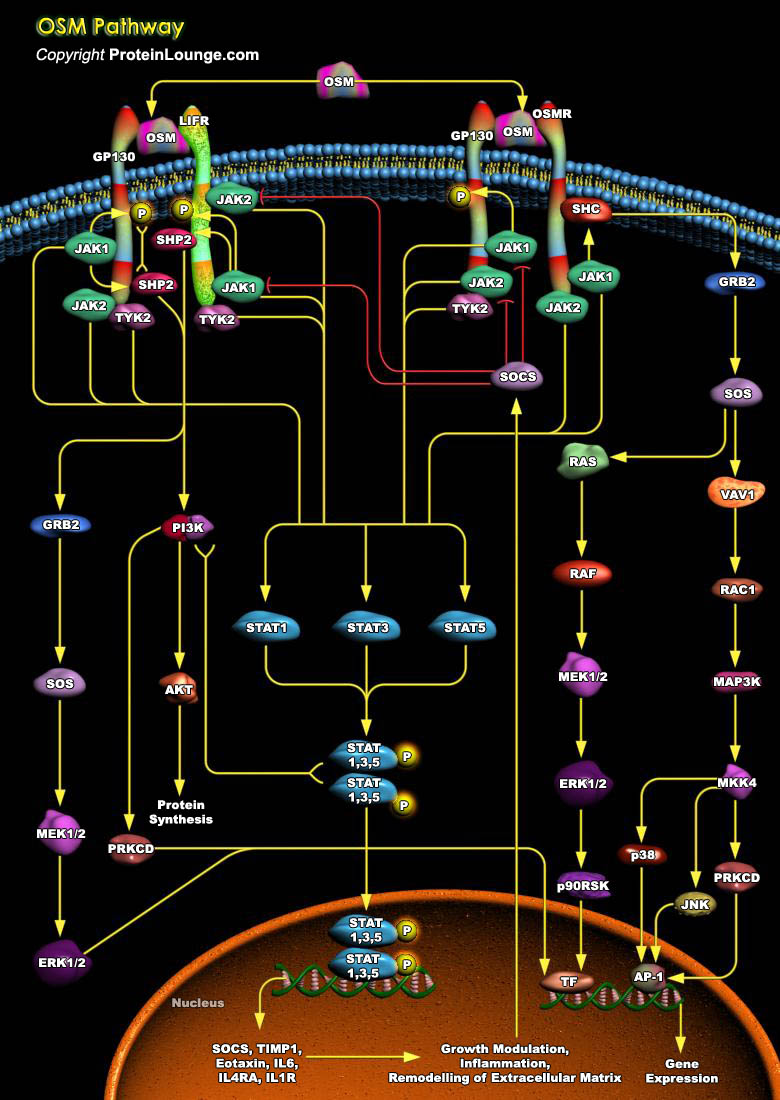
Oncostatin M (OSM) is a 28-kDa pleiotropic cytokine belonging to the Interleukin-6 (IL-6)familythat bears closest homology toleukemia inhibitory factor (LIF). Human OSM (hOSM) is a252 amino acid precursor polypeptide that is mainly expressed by neutrophils, activated macrophages, dendritic cells and T-cells. OSM appears to play important role in diverse biological processes like development of blood cells, differentiation, inflammation, growth, metabolism and neuroprotection. OSM signals through heterodimers of transmembrane receptor GP130 with either leukemia inhibitory factor receptor (LIFR) or oncostatin M receptor (OSMR). After recruiting therequired receptors, OSM signals through JAK/STAT, MAPK and PI3K/AKT pathways (Ref.1 and 2).JAK/STAT pathway is the major[..]
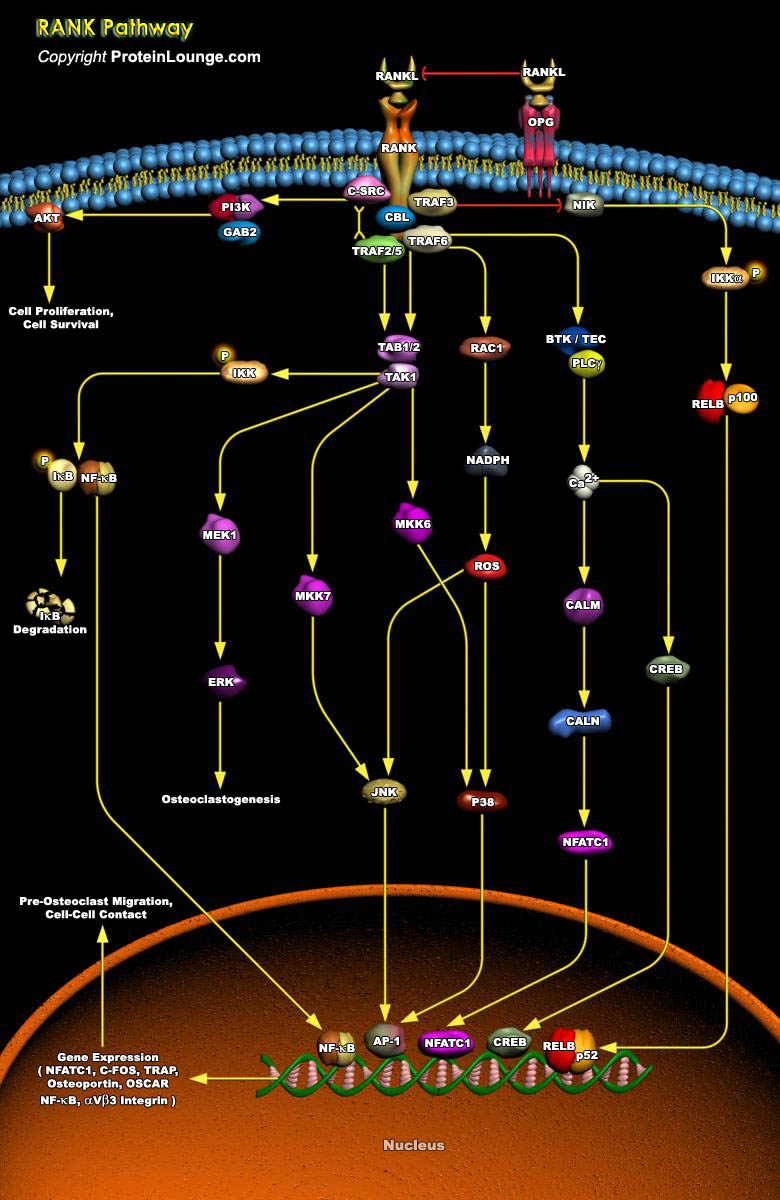
Bone remodeling and homeostasis is an essential function that regulates skeletal integrity throughout adult life in higher vertebrates and mammals. The structural and metabolic integrity of bone is maintained through the dynamic process of bone remodeling that results from the coordinate action of bone resorption by osteoclasts and the formation of new bone by osteoblasts. Regulation of bone remodeling occurs through multiple mechanisms that ultimately converge on the interaction of osteoclasts or their precursors with osteoblasts and bone marrow stromal cells. Key factors supplied by the stromal environment are cytokines such as IL1, IL11, IL17, CSF1 (Colony Stimulating Factor-1) and the TNF receptor ligand family, RANKL (Receptor Activator of NF-kappaB Ligand; also[..]
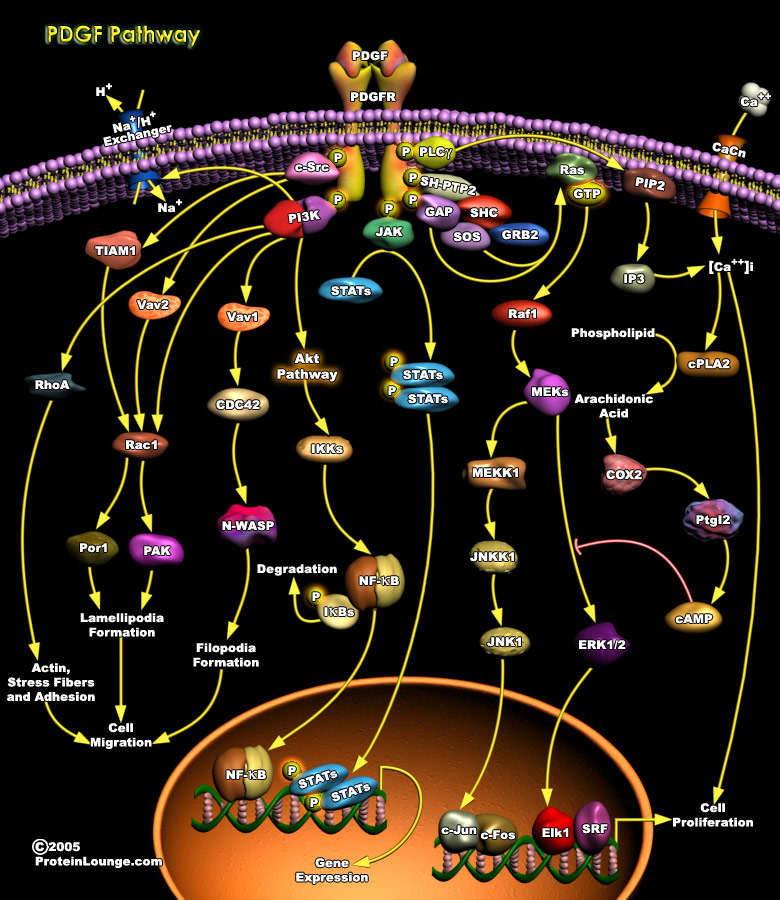
PDGF (Platelet-derived growth factor) isoforms stimulate growth, survival and motility of mesenchymal cells and certain other cell types. They have important functions during embryonal development and in the control of tissue homeostasis in the adult. Overactivity of PDGF signaling is associated with the development of certain malignant diseases, as well as non-malignant diseases characterized by excessive cell proliferation. The PDGF isoforms are synthesized as precursor molecules with signal sequences, precursor sequences and growth factor domain. After dimerization, the isoforms are proteolytically processed to their active forms which bind to the receptors. The extracellular parts of the receptors contain 5 Ig-like domains; ligand binding occurs preferentially to[..]
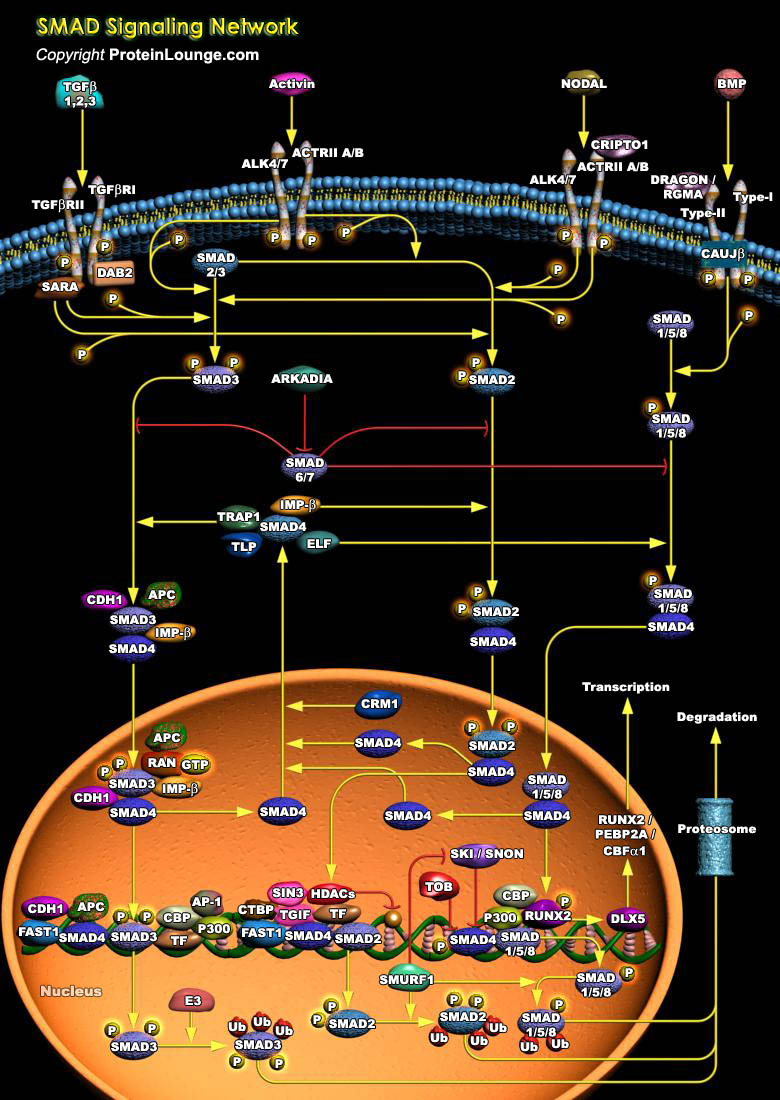
TGF-Beta (Transforming Growth Factor-Beta) superfamily of secreted polypeptide growth factors play an important role in a variety of pathophysiologic processes, including angiogenesis, vascular remodeling, atherogenesis and in regulating cellular responses such as growth, proliferation, differentiation, migration, adhesion, survival, and specification of developmental fate. Apart from TGF-Beta, the superfamily also includes the Activins, NODAL and the BMPs (Bone Morphogenetic Proteins). These factors signal through heteromeric complexes of Type-II and Type-I serine-threonine kinase receptors, which activate the downstream SMAD (Sma and Mad Related Family) signal transduction pathway (Ref.1, 2 & 3).Based on their structures and known functional roles, the mammalian[..]
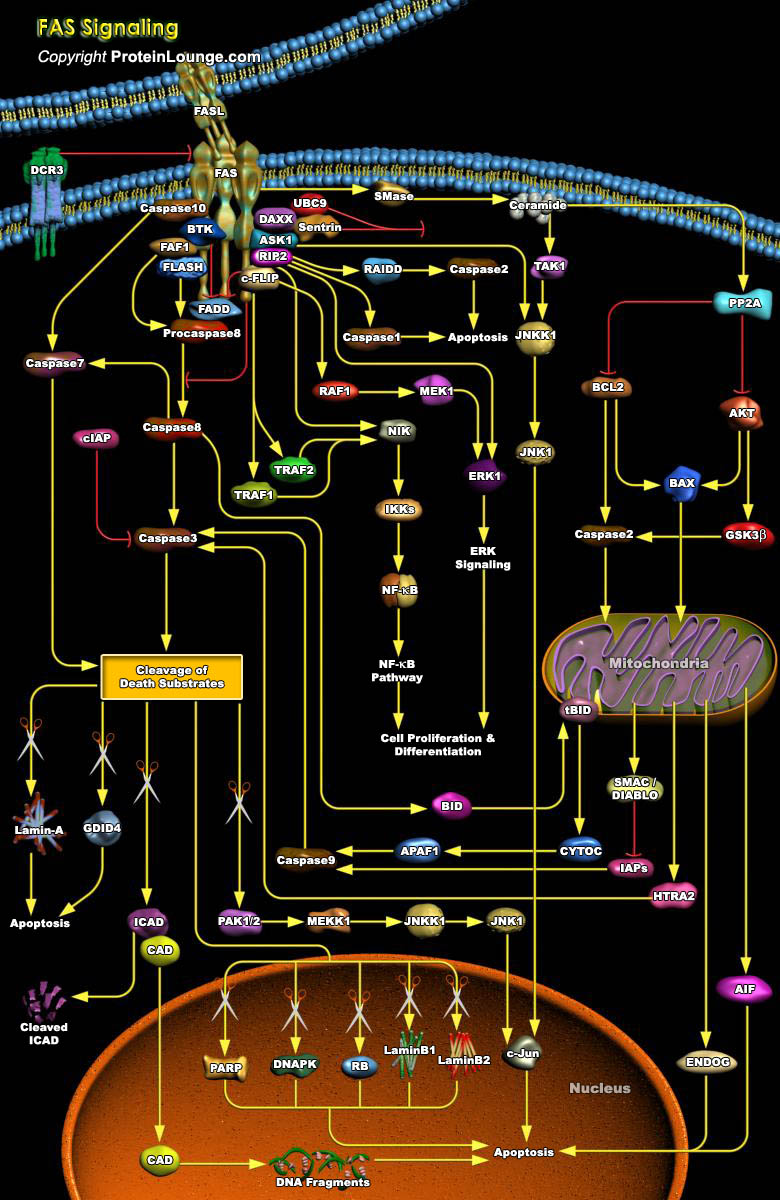
Fas (also called Apo1 or CD95) is a death domain-containing member of the TNFR (Tumor Necrosis Factor Receptor) superfamily. It has a central role in the physiological regulation of Programmed Cell Death and has been implicated in the pathogenesis of various malignancies and diseases of the immune system. Although the FasL (Fas Ligand)-Fas system has been appreciated mainly with respect to its death-inducing function, it also transduces proliferative and activating signals through pathways that are still poorly defined. The Fas Receptor induces an apoptotic signal by binding to FasL expressed on the surface of other cells. Fas is a Type-I transmembrane protein, where as FasL a Type-II Transmembrane protein of TNF family and can be shed in a soluble form by action of[..]
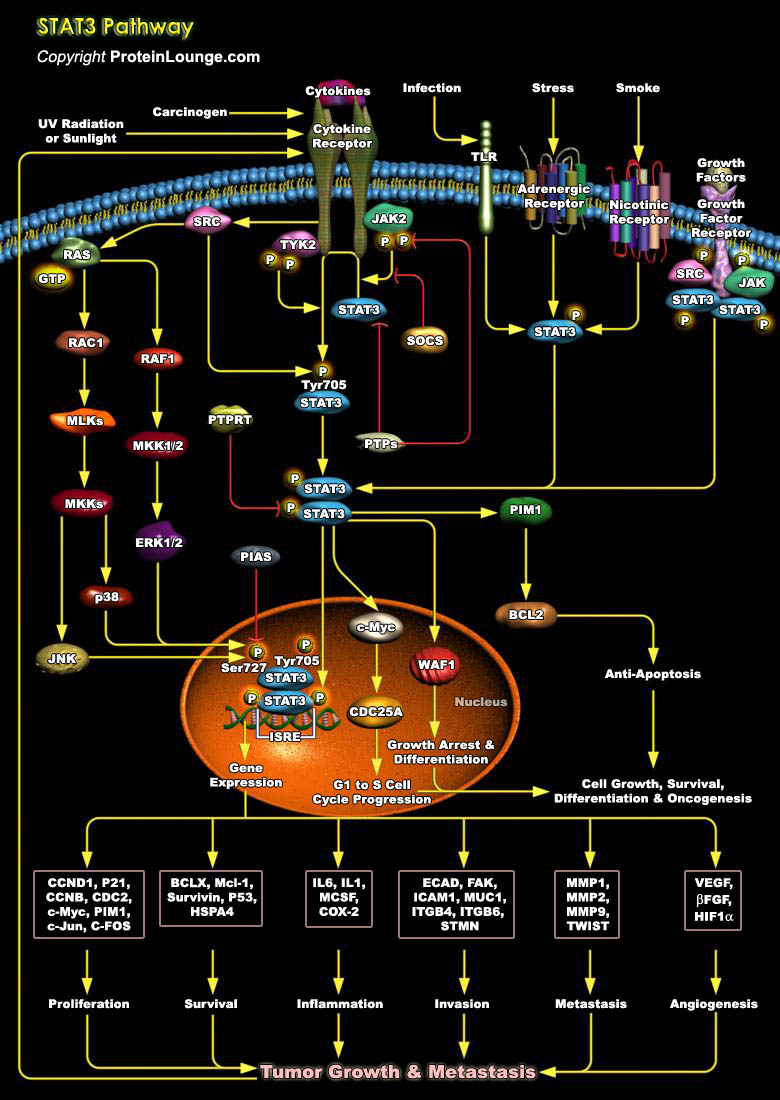
STATs (Signal Transducers and Activators of Transcription) are a family of cytoplasmic proteins with SH2 (Src Homology-2) domains that act as signal messengers and transcription factors that mediate many aspects of cellular immunity, proliferation, apoptosis and differentiation. The STAT family comprises seven members namely, STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6. They range in size from 750 to 850 amino acids and the entire STAT family can be divided into two groups based on their functions. The first group consists of STAT2, STAT4, and STAT6, which are activated through a small number of cytokines and are involved in T-cell development and IFN-γ signaling. The second group includes STAT1, STAT3, and STAT5, which are activated in different[..]
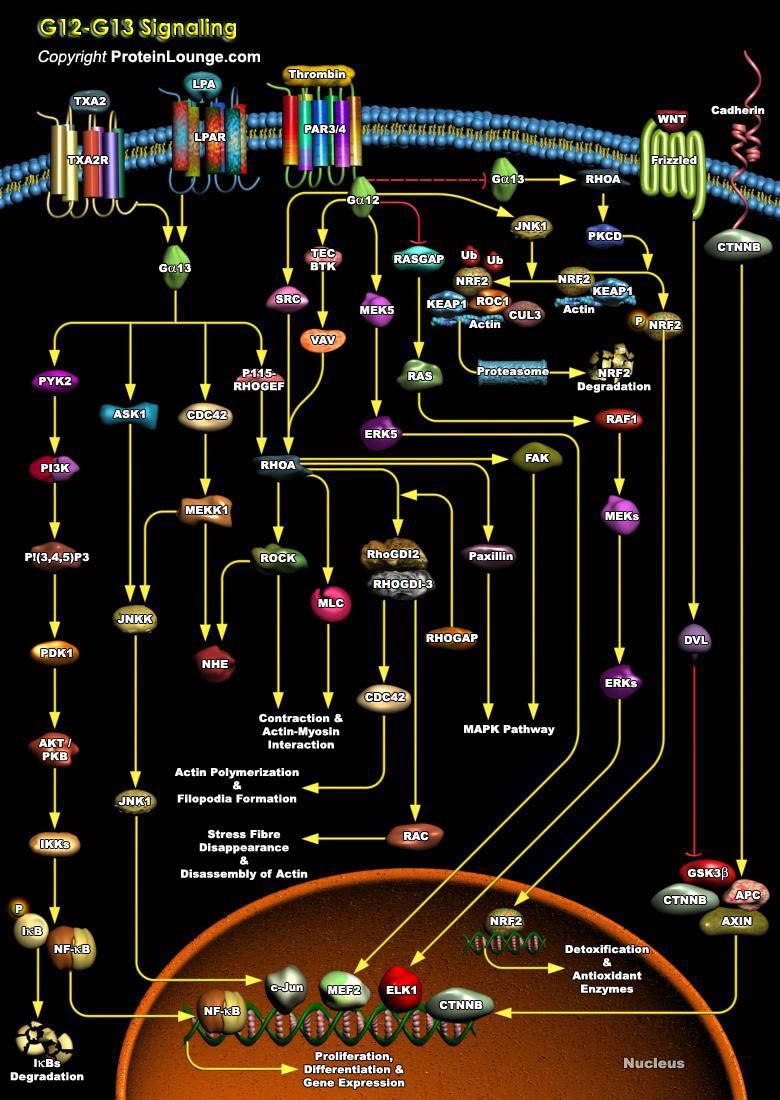
The G12 subfamily of heterotrimeric G proteins, comprised of the Alpha-subunits G-Alpha12 and G-Alpha13, has been implicated as a signaling component in cellular processes ranging from cytoskeletal changes to cell growth and oncogenesis. Activated G-Alpha12 and G-Alpha13 have a molecular weight of 43,000 kDa and they show more than 66% amino acid identity. They stimulate mitogenic signaling pathways leading to the oncogenic transformation of fibroblast cell lines. Recent analyses have indicated that G-Alpha12 and G-Alpha13 regulate cytoplasmic as well as nuclear signaling events through downstream targets such as Ras-, Rac-, Rho-, and CDC42 (Cell Division Cycle-42) dependent pathways, leading to cytoskeletal reorganization and to the activation of MAPK[..]
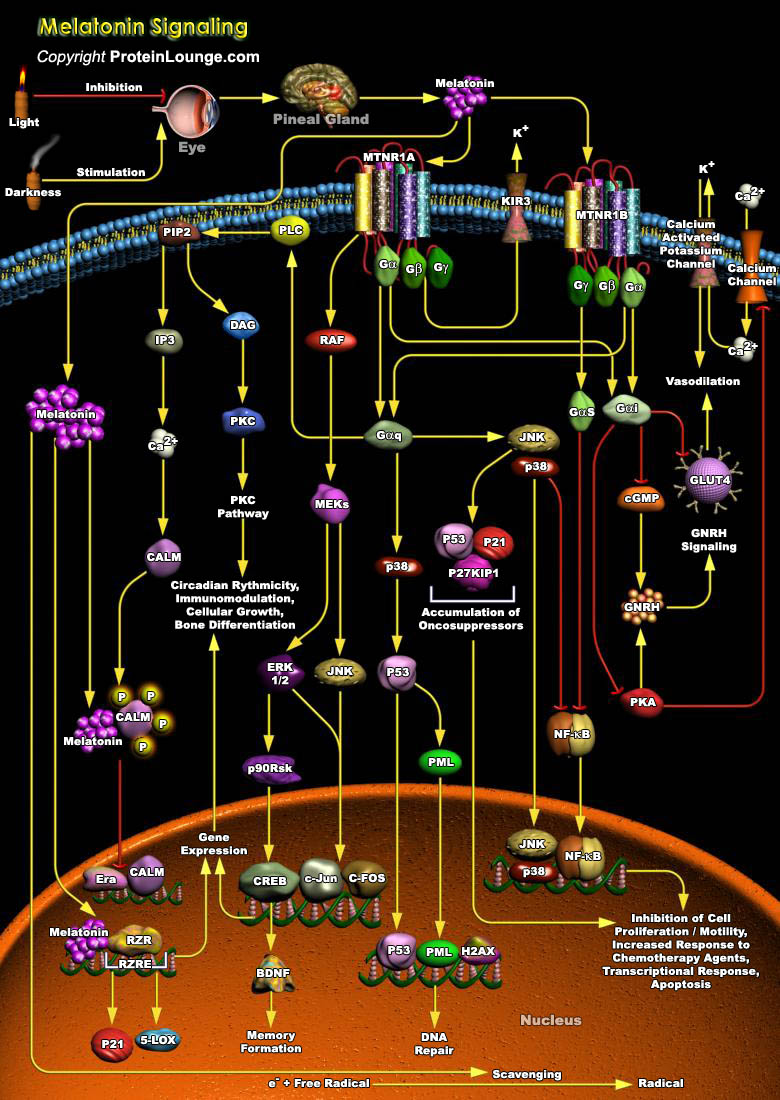
In mammals, the circadian system is comprised of three major components: the lateral eyes, the hypothalamic SCN (Suprachiasmatic Nucleus) and the pineal gland. The SCN harbours the endogenous oscillator that is entrained everyday to the ambient lighting conditions via retinal input. Among the many circadian rhythms in the body that are driven by SCN output, the synthesis of Melatonin (N-Acetyl-5 Methoxy-Tryptamine) in the pineal gland functions as a hormonal message encoding for the duration of darkness. Melatonin is a hormone secreted mainly by the pineal gland or epiphysis; it is also produced, but in much smaller quantities, by the retina. Melatonin is produced nocturnally, and is a neurochemical representation of time. Dissemination of this circadian[..]
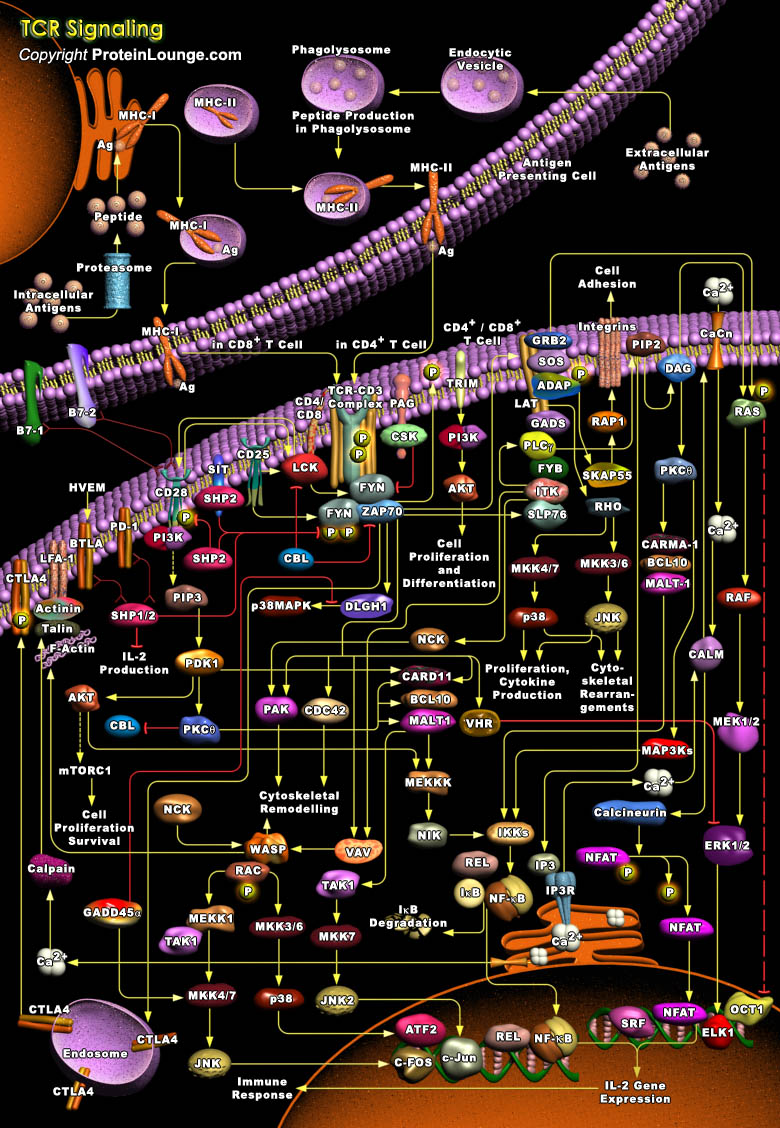
T-Cells are a subset of lymphocytes that play a large role in the immune response. The TCR (T-Cell Receptor) is a complex of integral membrane proteins that participates in the activation of T-Cells in response to the presentation of antigen. Stimulation of TCR is triggered by MHC (Major Histocompatibility Complex) molecules on Antigen Presenting Cells that present antigen peptides to TCR complexes and induce a series of intracellular signaling cascades. Engagement of the TCR initiates positive (signal-enhancing) and negative (signal-attenuating) cascades that ultimately result in cellular proliferation, differentiation, cytokine production, and/or activation-induced cell death. These signaling cascades regulate T-Cell development, homeostasis, activation, acquisition[..]
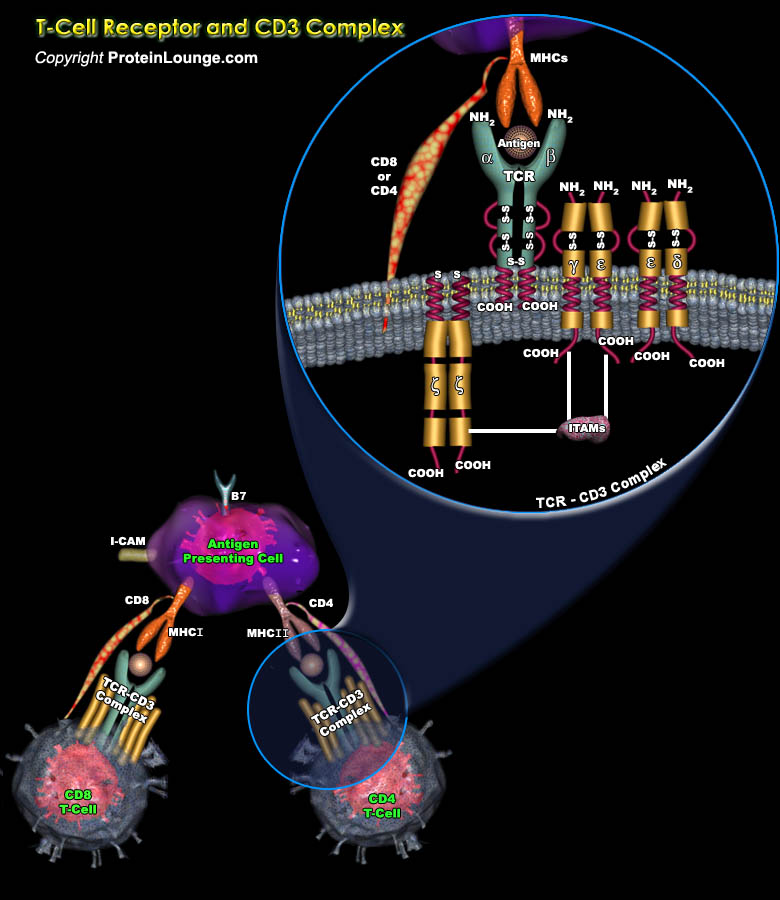
The lymphocyte population is mainly made up of the T-lymphocytes (Thymus-Derived Lymphocytes), B-lymphocytes (bone-marrow-derived), and the NK cells (Natural-Killer Cells). T-lymphocytes mediating the cellular immunity, along with B lymphocytes mediating humoral immunity, provide adaptive immunity, which work in close collaboration with the innate immune system. B-lymphocytes mature in the bone marrow itself, while the T-lymphocytes require the thymus to mature, before being deployed to the peripheral lymphoid organs for further antigen-mediated differentiation. These cells are responsible for antibody production, direct cell-mediated killing of virus-infected and tumor cells, and regulation of the immune response (Ref.1).T cells are key mediators of the adaptive[..]
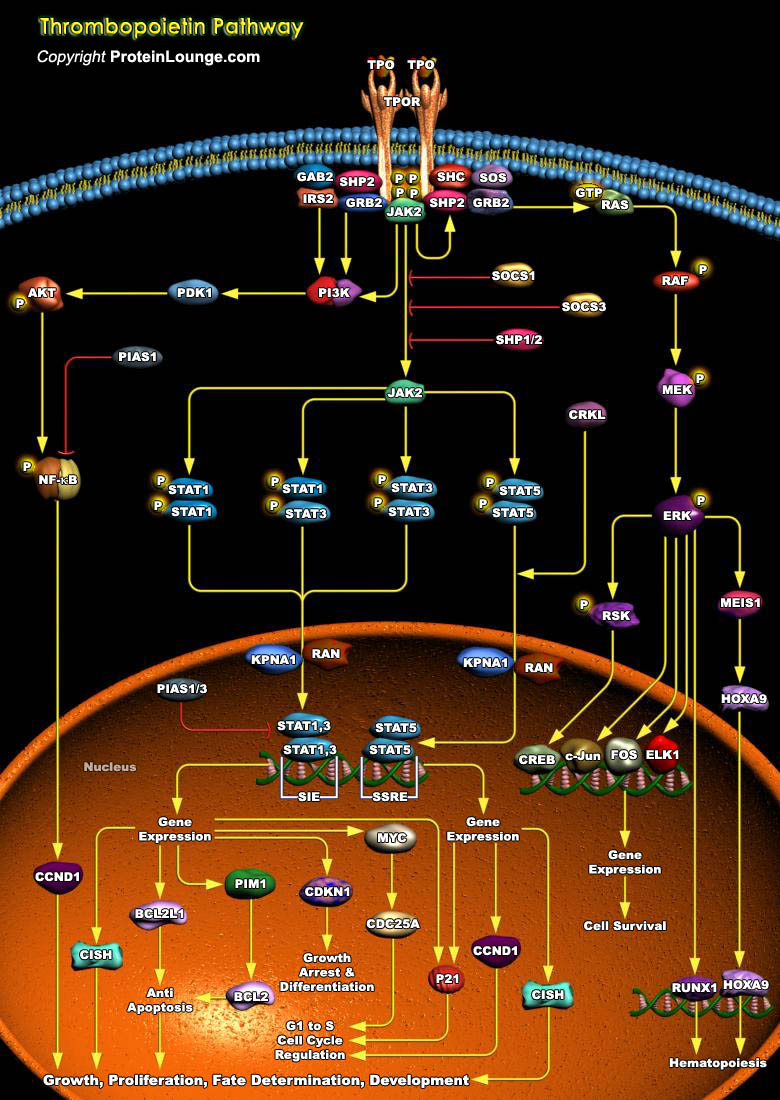
Throughout adult life, all blood cells are constantly regenerated from a small pool of hematopoietic stem cells. A single purified stem cell injected into a lethally irradiated host is sufficient to reconstitute all lineages, demonstrating pluripotency and an enormous regenerative potential. Genes encoding secreted growth factors, signal transduction molecules and nuclear proteins regulate this process. The helical cytokine family comprises structurally related secreted proteins that play an important role as hematopoietic growth factors. Their cognate receptors are single transmembrane proteins that constitute the so-called cytokine receptor superfamily. One such ligand-receptor pair is TPO (Thrombopoietin), also known as the c-MPL ligand or MGDF (Megakaryocyte Growth[..]

