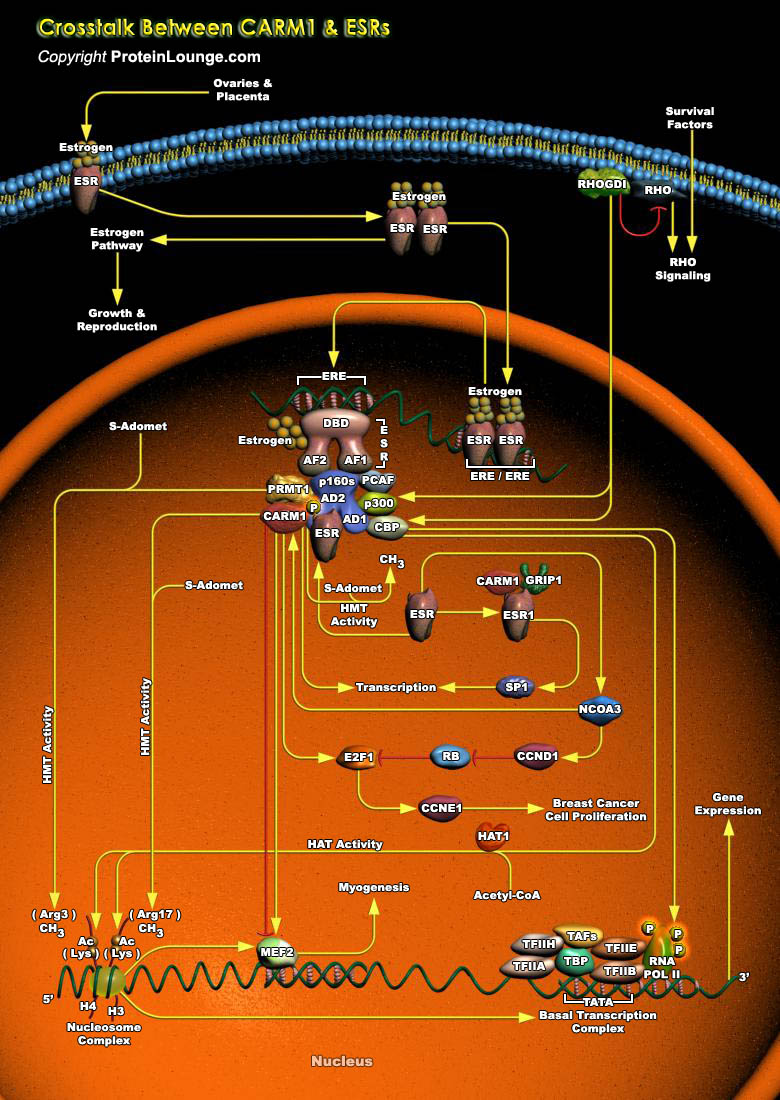
The ESRs (Estrogen Receptors) are ligand-dependent transcription factors and are important Nuclear Hormone Receptors that act as regulators of cell growth, differentiation and malignant transformation. Transcriptional activation by ESRs is accomplished through specific and general cofactor complexes that assemble with the receptor at target promoters to regulate transcription. The chief ligand for ESR is the ovarian steroid hormone Estrogen, which has a primary role in the establishment and maintenance of reproductive function (Ref.1). Naturally occurring forms of Estrogen are Estradiol, Estriol, and Estrone. Estradiol is the most commonly occurring form of Estrogen in non-pregnant women. Binding of Estrogen to the ESR promotes a conformational change in the receptor[..]
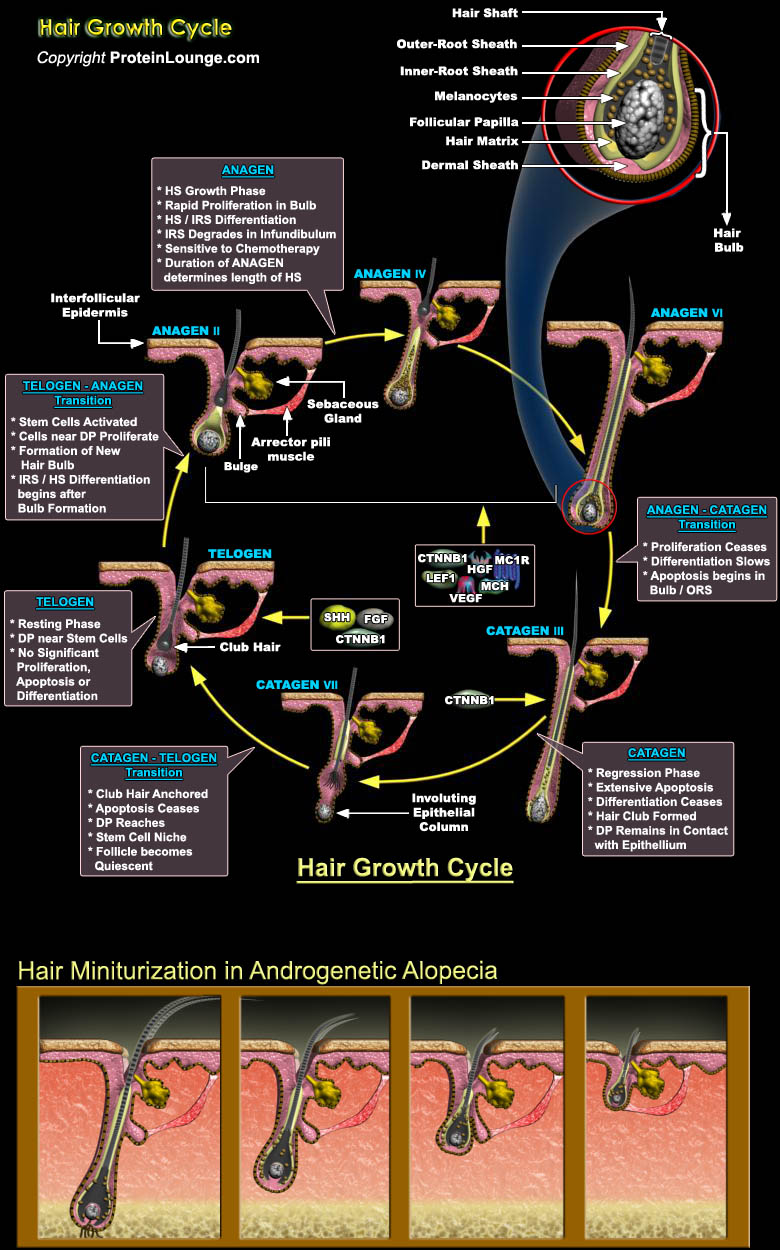
The hair follicle is a three-dimensional tube, composed mainly of epithelial cells that protrude down through the epidermis and dermis of the skin, enveloping at its base the mesenchyme-derived dermal papilla. These hair follicle acts as a sensory organ and immunologic sentinel for the skin. Hairs detect mechanical stimuli above the surface of the skin, and the slightest bend in a hair activates Neuroreceptors in the follicle, relaying important sensory information to the Nervous System. The Langerhans' cells (Dendritic Antigen-Presenting Cells) at the opening of the follicle detect surface pathogens and activate the immune system. The hair follicle has a complex immunologic profile, with immunologically "privileged" matrix at its base, and a complement of[..]
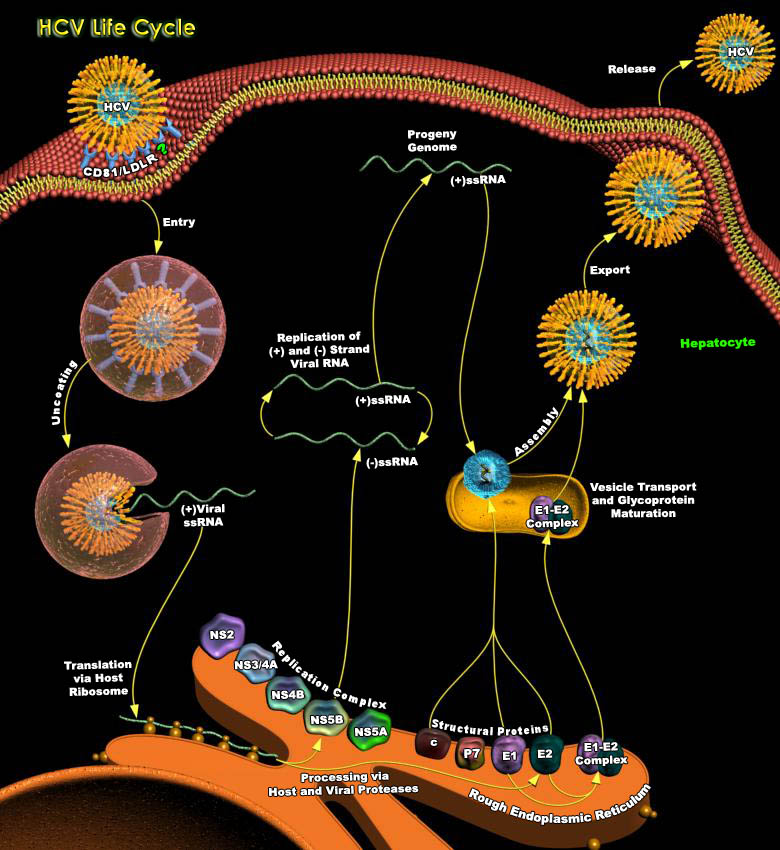
Hepatitis-C Virus (HCV) belongs to the Flaviviridae family and is the leading cause of chronic liver disease globally. It is estimated to infect about 170 million people around the world (WHO, 1997). Chronic HCV infection frequently leads to liver fibrosis and cirrhosis, and is associated with the occurrence of hepatocellular carcinoma. Acute infection occurs in only a few patients. In most cases the virus results in chronic infection taking 10–20 years before the emergence of liver disease, which is often accompanied by only mild or vague symptoms. Despite the seemingly benign onset of the disease, a significant number of patients with chronic hepatitis develop cirrhosis and its complications.HCV infects only humans and chimpanzees; there are no small-animal[..]
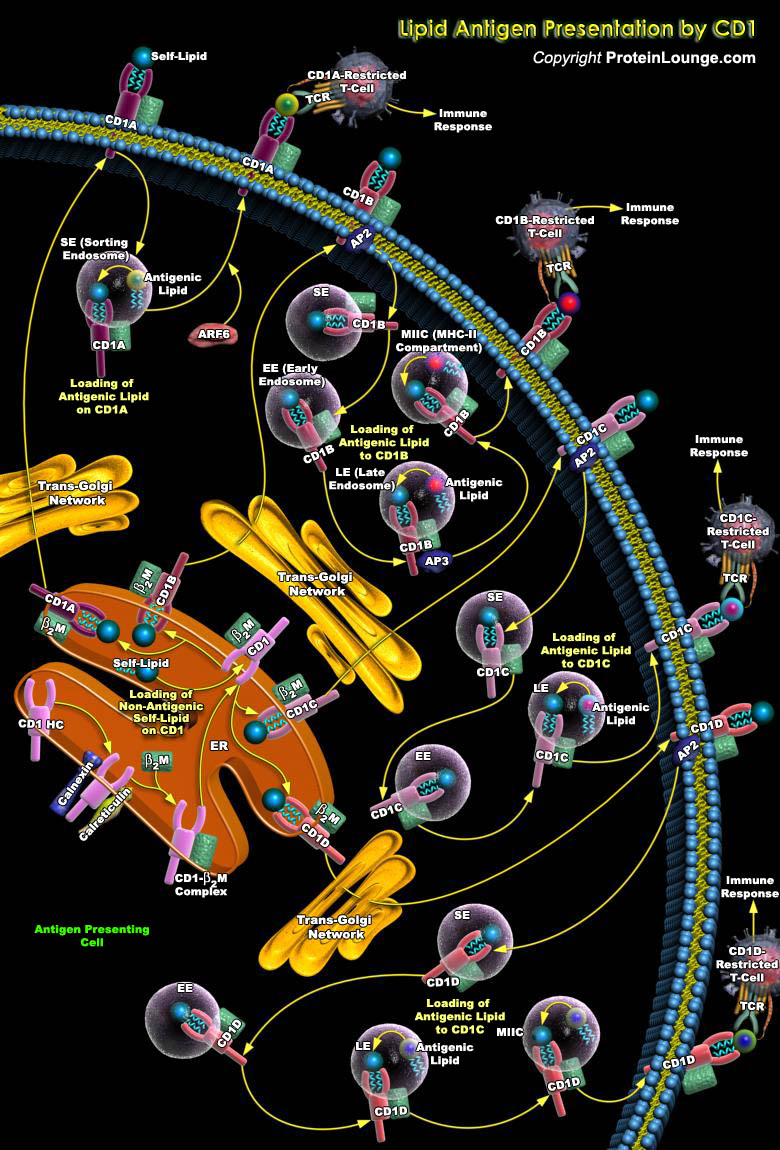
The CD1 (Thymocyte Antigen CD1) antigen presentation system presents lipid and glycolipid antigens to effector T-Cells, which have diverse roles in Antimicrobial responses, Antitumor immunity and in regulating the balance between Tolerance and Autoimmunity. CD1, a conserved family of MHC (Major Histocompatibility Complex)-like glycoproteins in mammals, specializes in capturing lipid rather than peptide antigen for presentation to T-lymphocytes (Ref.1). The CD1 family consists of five isoforms of non-polymorphic lipid antigen-presenting molecules, which are classified into two groups: Group-I (CD1A, CD1B, CD1C, and CD1E) and Group-II (CD1D), based on similarities between nucleotide and amino acid sequences. Topologically, they resemble the classical peptide[..]
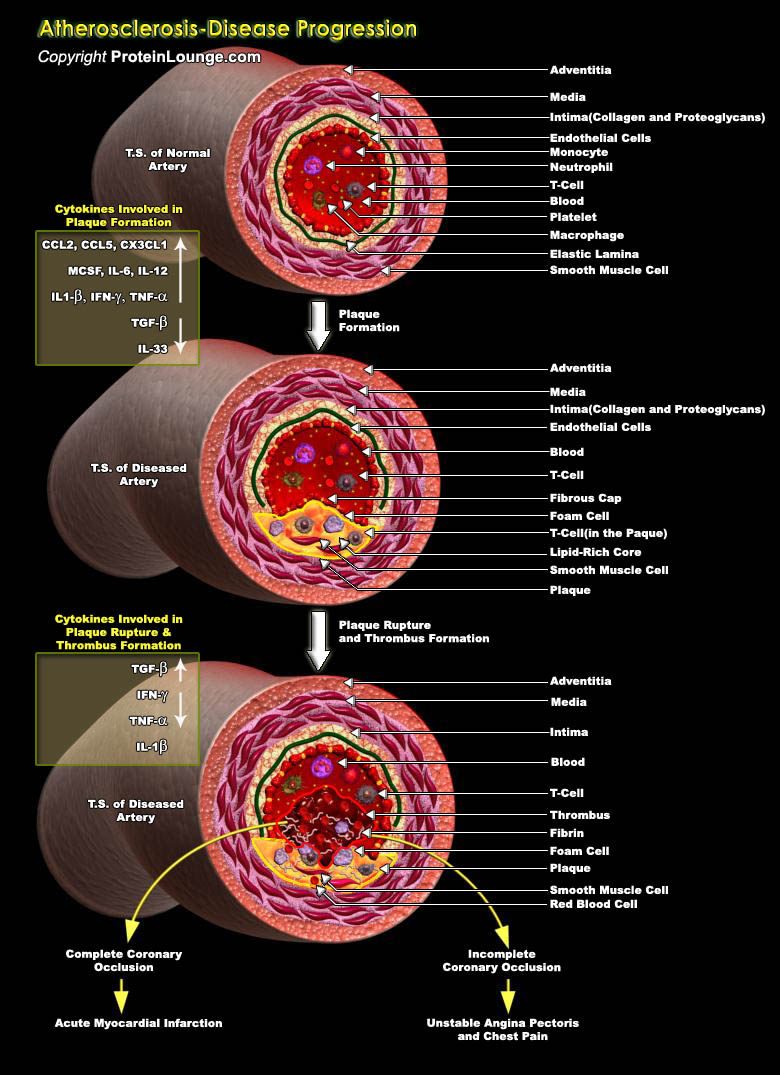
Atherosclerosis is a chronic disease, the prevalence of which has increased steadily as the population ages. Vascular injury is believed to be critical initiating event in pathogenesis of spontaneous atherosclerosis. Economic development and urbanization have promoted habits of diet rich in saturated fat and diminished physical activity, which favors atherosclerosis. Traditionally two types of atherosclerosis are described, spontaneous and accelerated. Accelerated atherosclerosis mainly occurs in patients after heart transplant, CABG (coronary artery bypass graft), and PTCA (percutaneous transluminal coronary angioplasty) [Ref.1]. The normal artery contains three layers. The inner layer, the tunica intima, is lined by a monolayer of endothelial cells that is in contact[..]
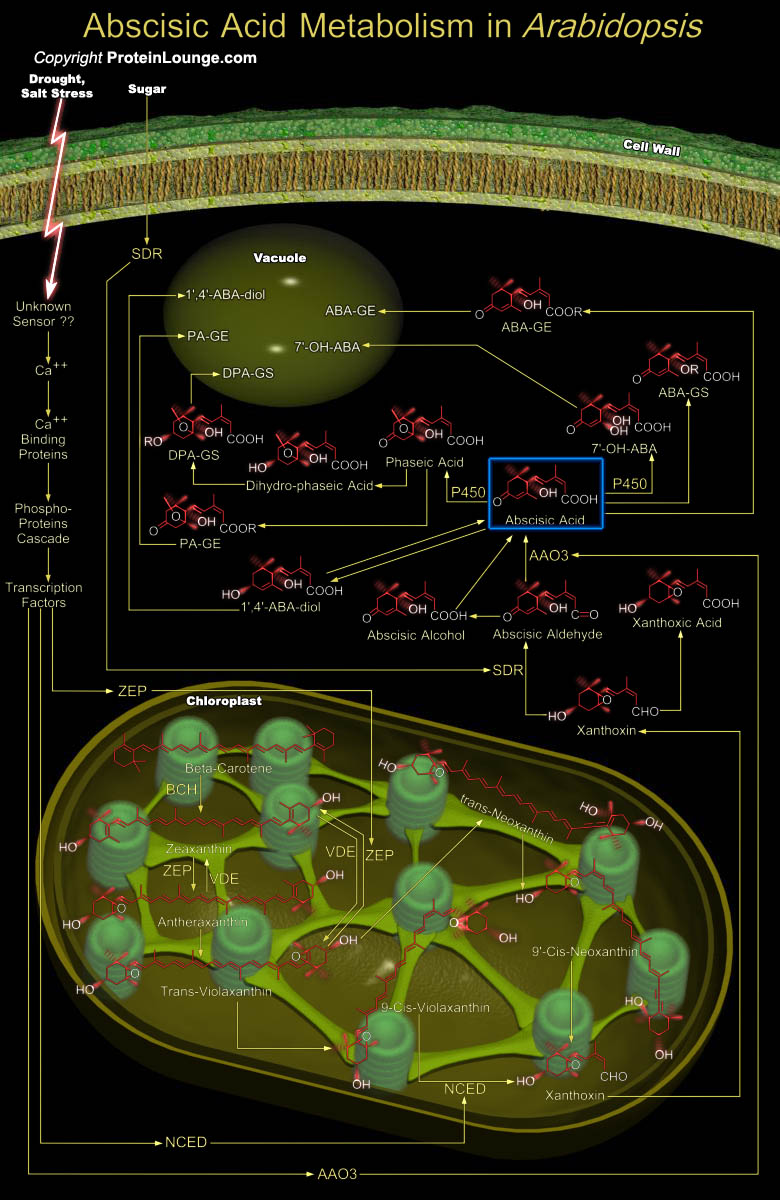
ABA (Abscisic Acid) is a plant hormone that plays important role during many phases of the plant life cycle, including seed development and dormancy, embryo maturation, cell division and elongation, and in plant responses to various environmental stresses such as drought, salinity, cold, pathogen attack and UV radiation. However, despite the name, it does not appear to control Abscission directly; the presence of ABA in Abscising organs reflects its role in promoting senescence and/or stress responses, the processes preceding Abscission. ABA is a sesquiterpenoid (C15H20O4) with one asymmetric, optically active Carbon atom at C-1'f. The naturally occurring form is S-(+)-ABA; the side chain of ABA is by definition 2-cis,-4-trans. Trans, trans-ABA is biologically[..]
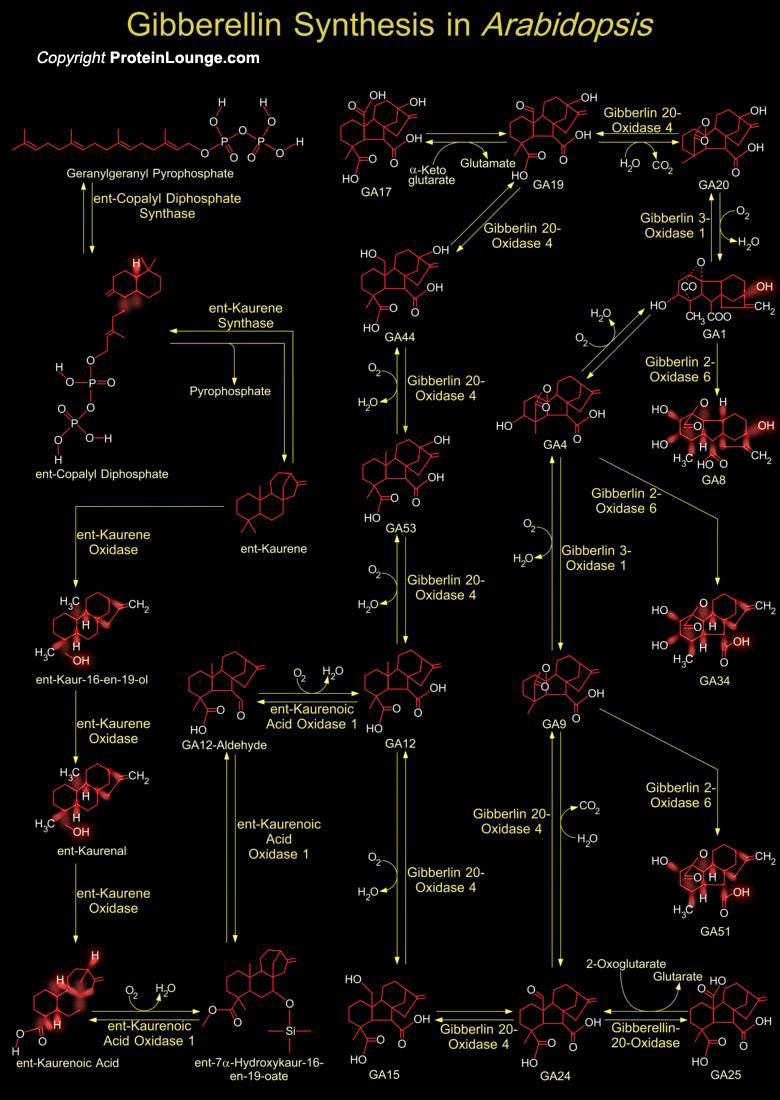
The GAs (Gibberellic Acids or Gibberellins) phytohormones play important roles in plant growth and development, promoting seed germination, elongation growth and reproductive development. GA is important for processes including seed germination, stem growth, and flower and fruit development. Gibberellins are modified in the endoplasmic reticulum and cytosol until they reach their biologically-active form. The gibberellins are named GA1 through GAn in order of discovery. Gibberellic acid, which was the first gibberellin to be structurally characterized, is GA3. There are currently 136 GAs identified from plants, fungi and bacteria.GAs is widespread and so far ubiquitous in flowering (Angiosperms) and non-flowering (Gymnosperms) plants as well as ferns. GA mediates[..]
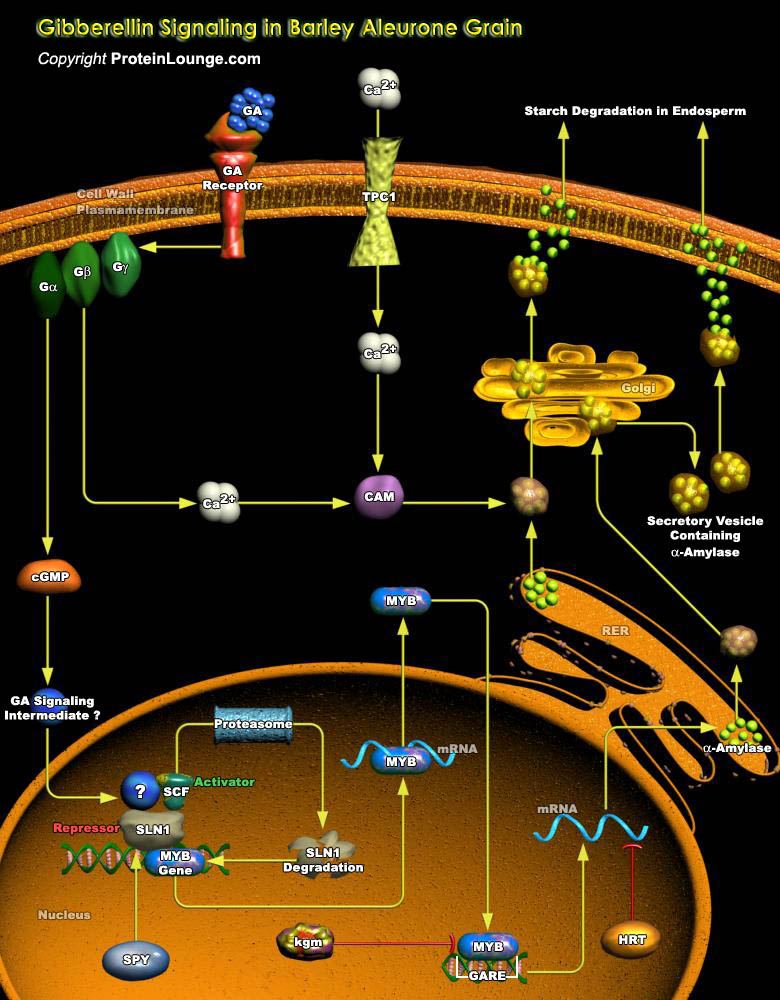
GAs (Gibberellins) are members of a large family of Diterpenoid compounds, which are essential for a number of processes, including Gene Expression in Cereal Aleurones, Seed Germination, Elongation, Growth, and Flowering. During the last four decades, Barley Aleurone has been a valuable system for studying GA regulation of gene expression. After germination, GAs are released from the Embryo into the Endosperm, triggering the expression of a number of genes encoding Hydrolytic enzymes in Aleurone cells. Many of these Hydrolytic enzymes, which include Alpha-Amylase, Proteases, and Cell Wall–degrading enzymes, are secreted and are responsible for digestion of the stored reserves in the starchy endosperm. The Signal transduction events leading from the Receptor to[..]
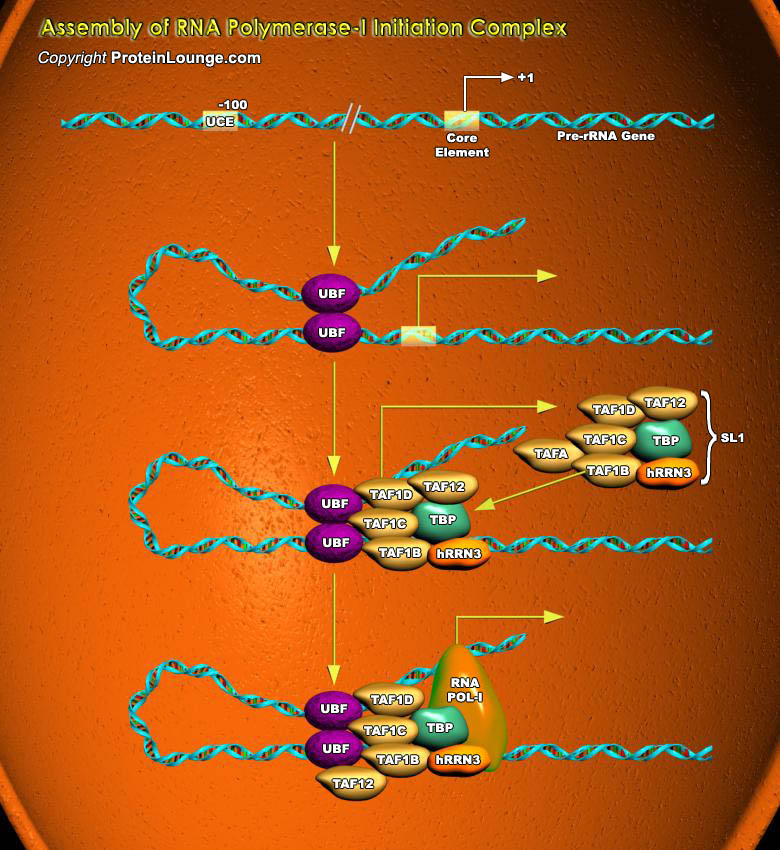
The nuclei of all eukaryotic cells contain three different RNA Polymerases, designated I, II and III. Like the DNA polymerase that catalyzes DNA replication, RNA Polymerases catalyze the formation of the phosphodiester bonds that link the nucleotides together to form a linear chain. The RNA polymerase moves stepwise along the DNA, unwinding the DNA helix just ahead of the active site for polymerization to expose a new region of the template strand for complementary base-pairing. In this way, the growing RNA chain is extended by one nucleotide at a time in the 5’-to-3’ direction. The substrates are nucleoside triphosphates (ATP, CTP, UTP, and GTP); as for DNA replication, a hydrolysis of high-energy bonds provides the energy needed to drive the reaction[..]
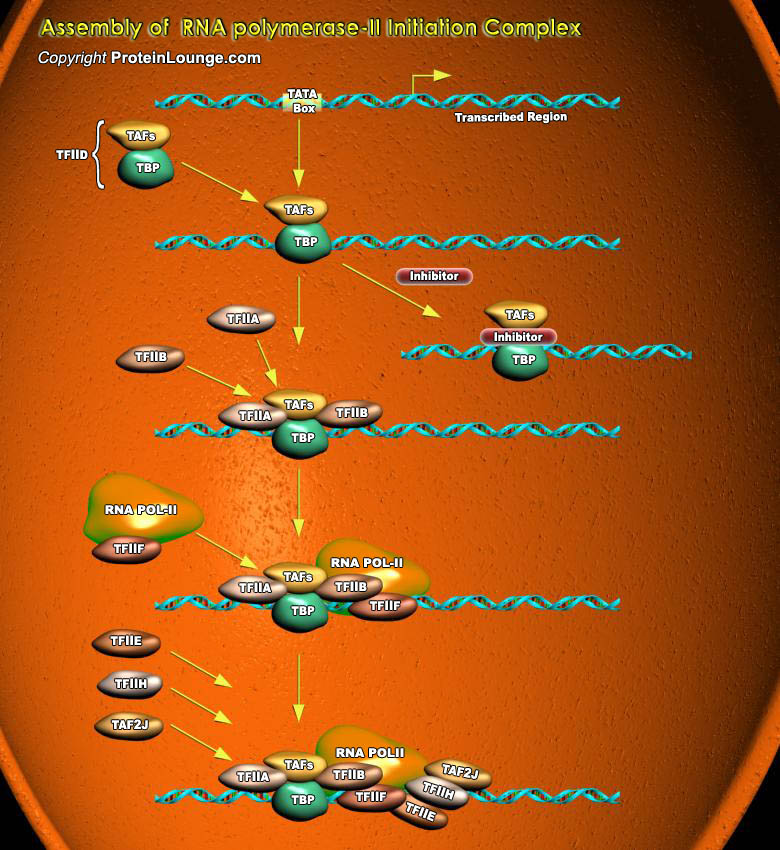
The nuclei of all eukaryotic cells contain three different RNA Polymerases, designated I, II and III. Like the DNA Polymerase that catalyzes DNA replication, RNA Polymerases catalyze the formation of the phosphodiester bonds that link the nucleotides together to form a linear chain. Each eukaryotic RNA Polymerase catalyzes transcription of genes encoding different classes of RNA. RNA Polymerase-II catalyzes transcription of all protein-coding genes; that is, it functions in production of mRNAs. RNA Polymerase-II also produces four snRNAs (small nuclear RNAs) that take part in RNA splicing.The eukaryotic polymerases do not directly recognize their core promoter sequences. The first step in complex formation at a promoter containing a TATA Box is binding of the factor[..]
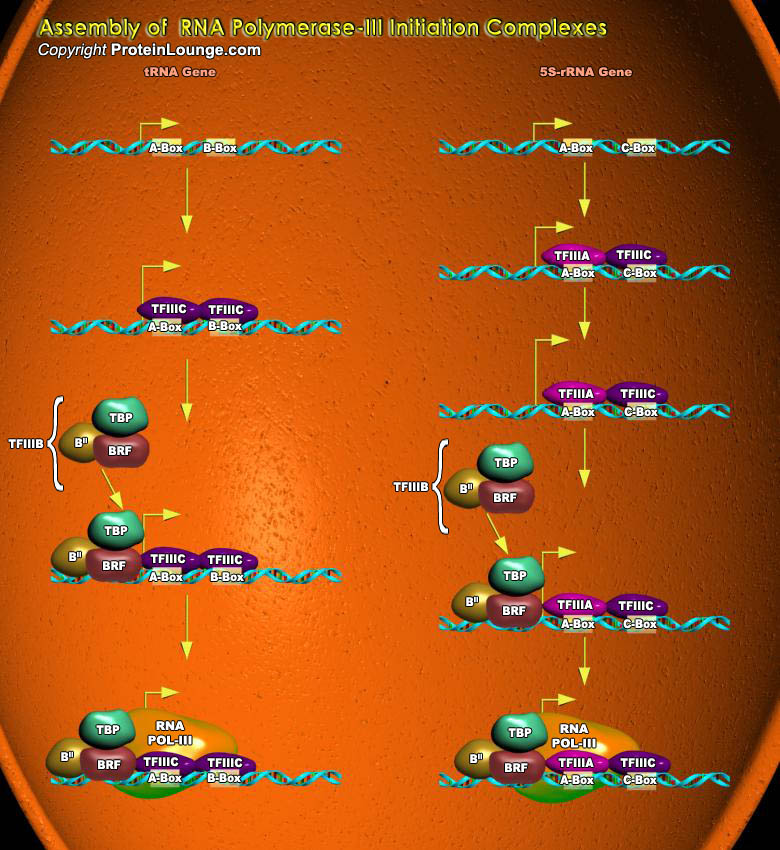
The nuclei of all eukaryotic cells contain three different RNA Polymerases, designated I, II and III. Like the DNA Polymerase that catalyzes DNA replication, RNA Polymerases catalyze the formation of the phosphodiester bonds that link the nucleotides together to form a linear chain. Each eukaryotic RNA Polymerase catalyzes transcription of genes encoding different classes of RNA. Transcription by RNA Polymerase-III produces small, stable RNAs including tRNAs, the 5S rRNA associated with the large ribosomal subunit, one of the snRNA (small nuclear RNAs) required for pre-mRNA splicing, and the 7S RNA associated with the signal recognition particle involved in secretion of proteins and the insertion of membrane-spanning proteins into cellular membranes. The func¬tions[..]
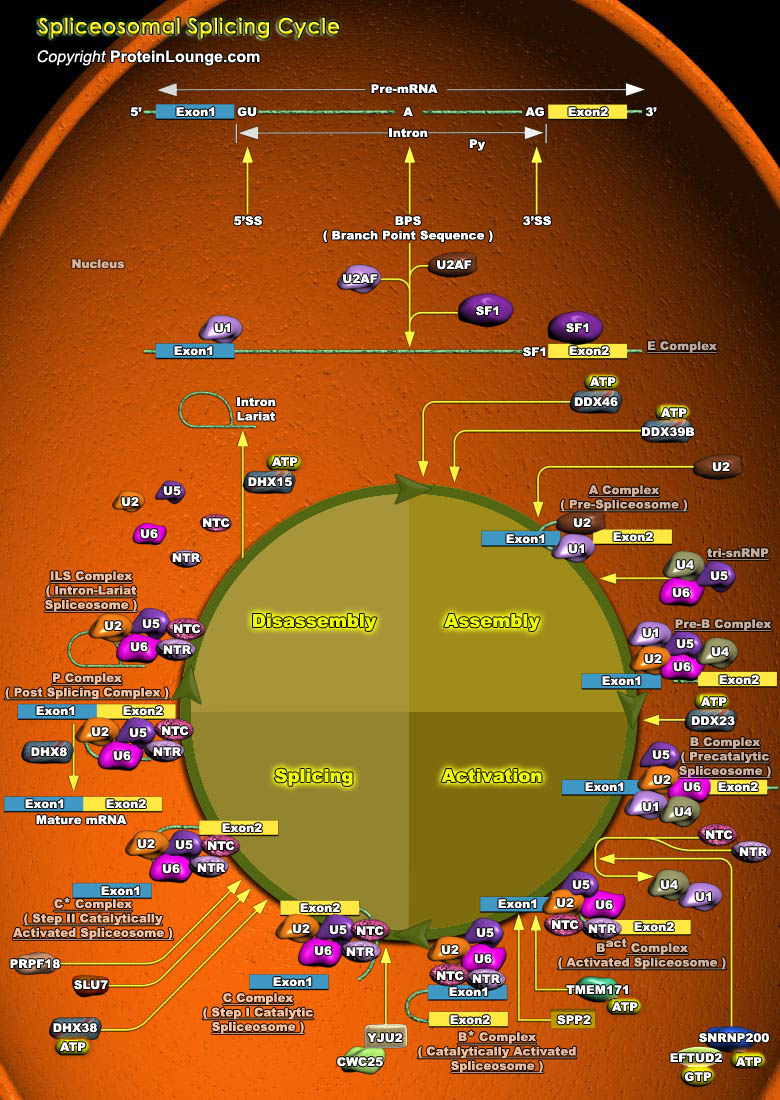
Splicing of pre-mRNA (pre-mRNA) is a complex mechanism where introns are removed, and exons are joined together to form a mature mRNA competent for translation. Pre-mRNA splicing is tightly regulated and its failure is linked to various tumors, pathologies of the endocrine system and neurodegenerative disorders. The discovery that introns are removed during splicing came from electron microscopy of RNA-DNA hybrids between adenovirus DNA and the mRNA encoding Hexon, a major virion capsid protein. For short transcription units, RNA splicing usually follows cleavage and polyadenylation of the 3’ end of the primary transcript. But for long transcription units containing multiple exons, splicing of exons in the nascent RNA usually begins before transcription of the[..]

