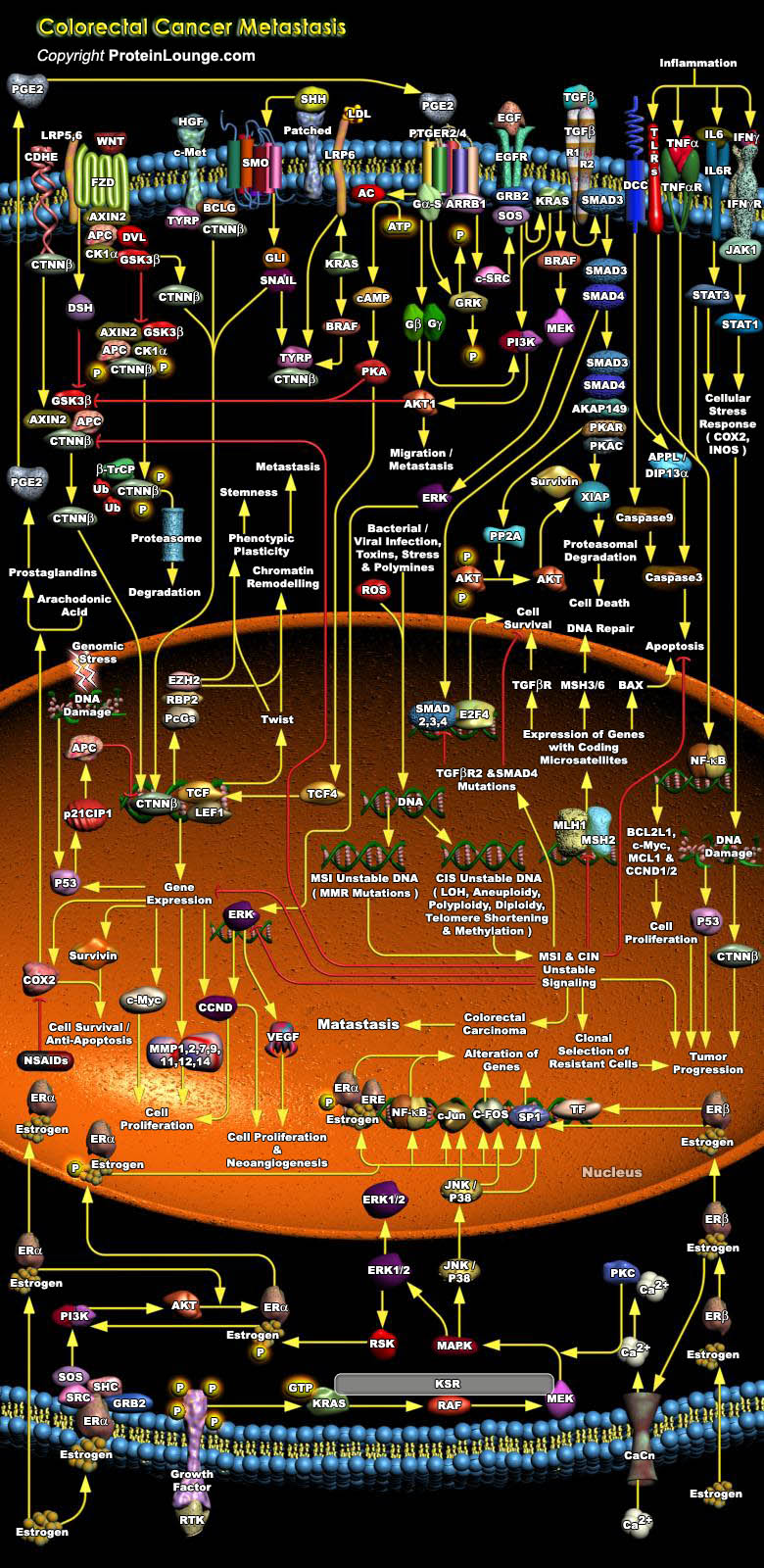
Colorectal cancer represents a relatively well-characterized tumorigenesis paradigm and colorectal carcinoma is one of the leading causes of cancer-related death. Colorectal cancer results from the accumulation of genetic alterations. Genomic instability creates a permissive state in which a potential cancer cell is allowed to acquire enough mutations to become a cancer cell. Several forms of genomic instability are common in colon cancer: MSI (Microsatellite Instability), CIN (Chromosome Instability), and chromosome translocations. MSI occurs in approximately 15% of colon cancers and results from inactivation of the MMR (Mismatch Repair) system secondary to either MMR gene mutations or hypermethylation of MMR gene promoters. It promotes tumorigenesis by generating[..]
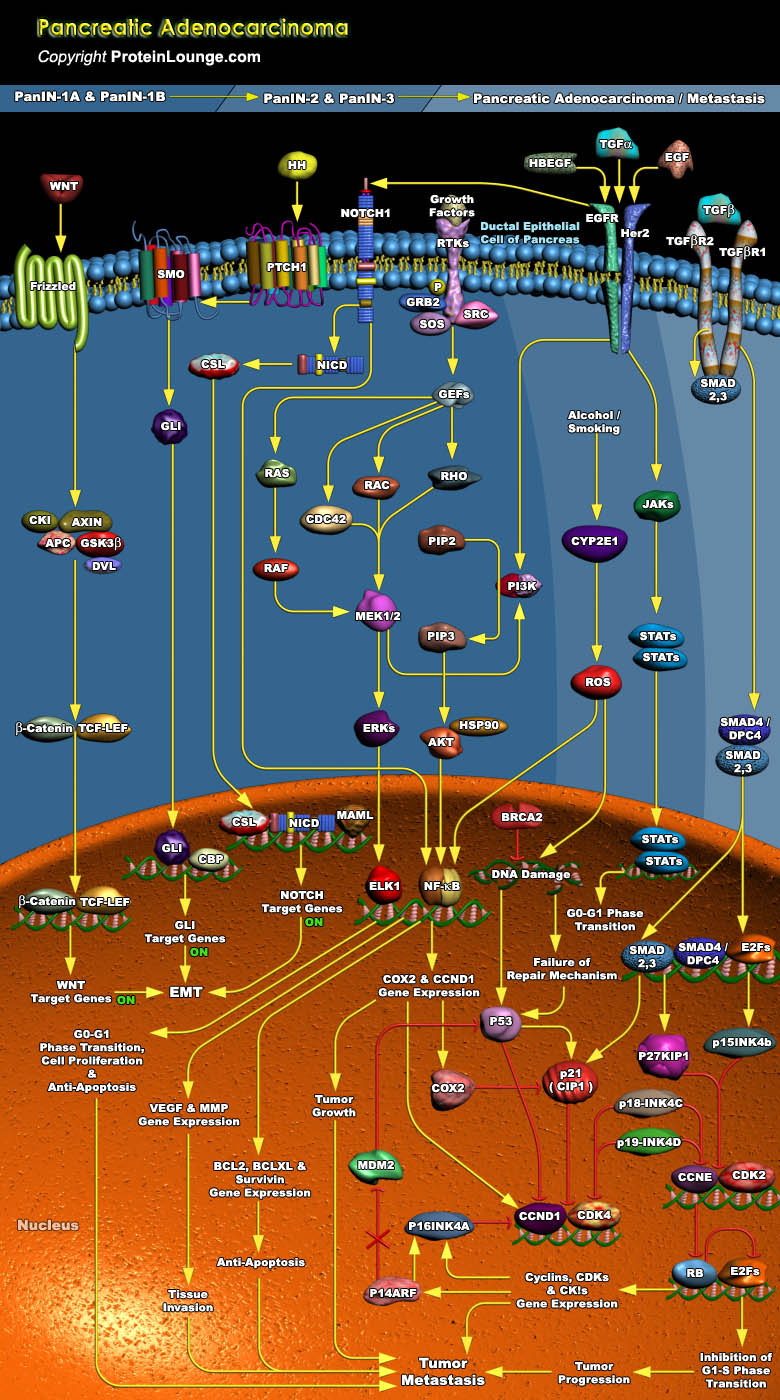
Pancreatic carcinoma is one of the most enigmatic and aggressive malignant diseases. Neoplasms of the pancreas encompass a wide spectrum of benign and malignant tumors. Pancreatic adenocarcinoma, the malignant neoplasm of the exocrine duct cells, accounts for more than ninety percent of all pancreatic tumors (Ref.1). Pancreatic ductal adenocarcinoma evolves from a progressive cascade of cellular, morphological and architectural changes from normal ductal epithelium through pre-neoplastic lesions termed PanIN (Pancreatic Intraepithelial Neoplasia). These PanIN lesions are in turn associated with somatic alterations in canonical oncogenes and tumor suppressor genes. Pancreatic cancer like many other tumors over-expresses the RTKs (Receptor Tyrosine Kinases), EGFR[..]
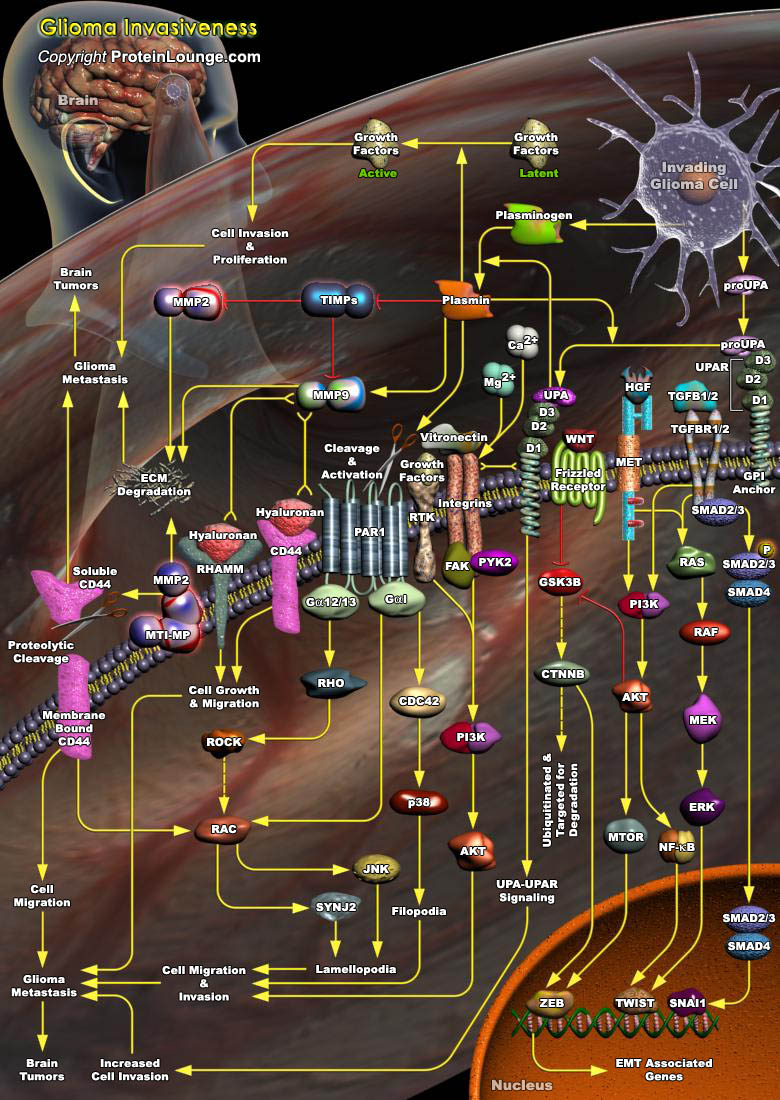
Gliomas are the most common intracranial malignant tumors in humans, and high-grade Gliomas in particular pose a unique challenge due to their propensity for proliferation and tissue invasion. The invasion of neoplastic cells into healthy brain tissue is a pathologic hallmark of Gliomas and contributes to the failure of current therapeutic modalities (surgery, radiation and chemotherapy). Transformed glial cells share the common attributes of the invasion process, including cell adhesion to ECM (Extracellular Matrix) components, cell locomotion, and the ability to remodel extracellular space. However, Glioma cells have the ability to invade as single cells through the unique environment of the normal CNS (Central Nervous System). The brain parenchyma has a unique[..]
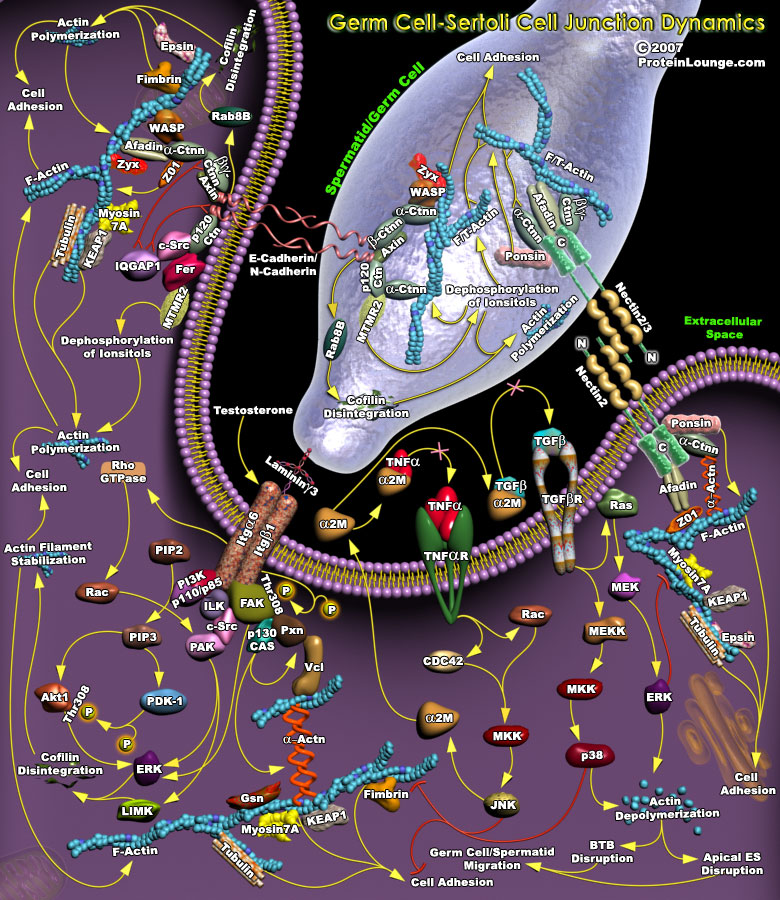
Sertoli-Germ (Spermatid)-cell interactions affect spermatogenesis at the molecular, cellular and biochemical levels. Germ cell movement within the epithelium is vital because germ cells, if induced to release into the tubule lumen prematurely, will be unable to fertilize the ovum. On the other hand, if germ cells are forced to remain attached to the seminiferous epithelium for a period of time longer than necessary to complete their development, they will degenerate and eventually be phagocytosed by Sertoli cells and for this, proper regulation of germ cell migration in the seminiferous epithelium and at blood-testes barrier (abbreviated as BTB) is essential (Ref.1). Spermatid/germ cell and sertoli cell interactions in the testis occur through specialized junctions[..]
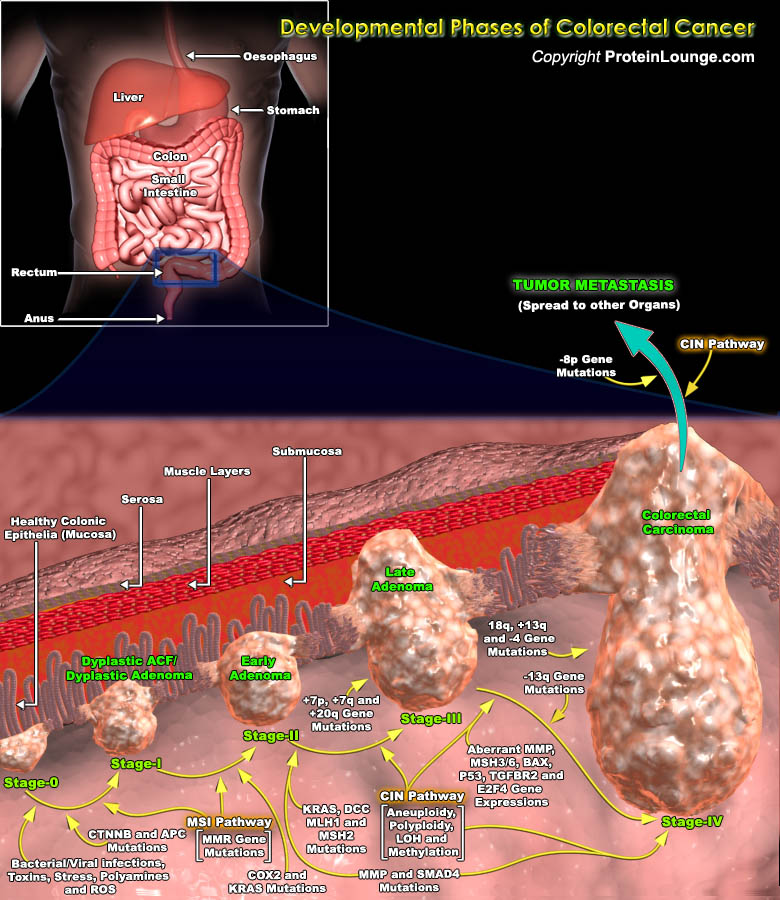
Colorectal tumors arise as a result of the mutational activation of oncogenes coupled with the mutational inactivation of tumor suppressor genes without a major role for gene amplification or rearrangement. These tumors affect the colon and rectum, and most colorectal cancers arise from adenomatous polyps. The development of colorectal neoplasms is characterized by an ordered series of events that are referred to as the “Dysplasia-Carcinoma Sequence” or “Adenoma-Carcinoma Sequence”. Mutations in at least four to five genes are required for the formation of malignant tumors and fewer changes only suffice for benign tumorigenesis. Although genetic alterations occur according to a preferred sequence, the total accumulation of changes, rather than[..]
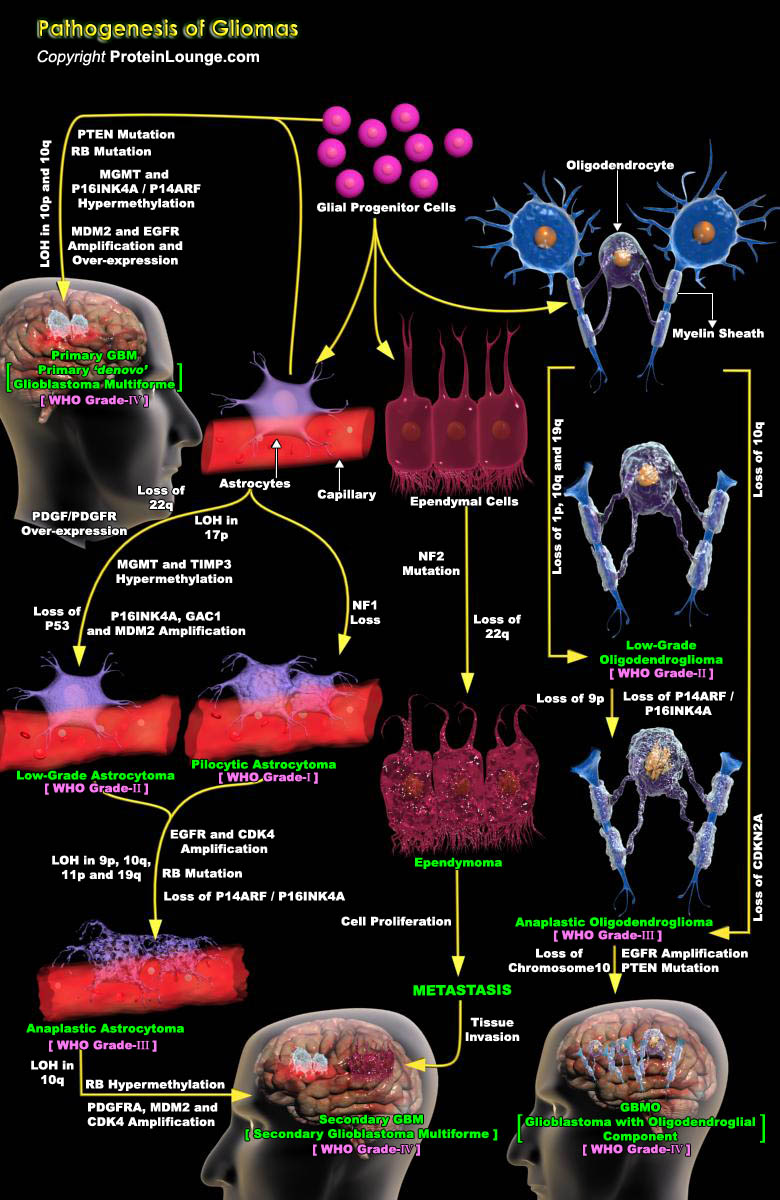
Tumors of the Central Nervous System (CNS) are devastating as they are difficult to treat and may cause grave disability or death. CNS Gliomas pose particularly difficult problems because of their tendency toward malignancy, rate of tumor spread, and the lack of effective therapy. Gliomas are the most common intracranial malignant tumors in humans (Ref.1). In vertebrates, the embryonic neural tube (neuroectoderm) gives rise to the main cell types of the CNS, including neurons, astrocytes, oligodendrocytes and ependymal cells. The neural crest derives from the dorsal lip of the neural tube, and its cells migrate extensively during embryonic development, giving rise to various tissues, including the Peripheral Nervous System (PNS). In both the CNS and PNS, the appearance[..]
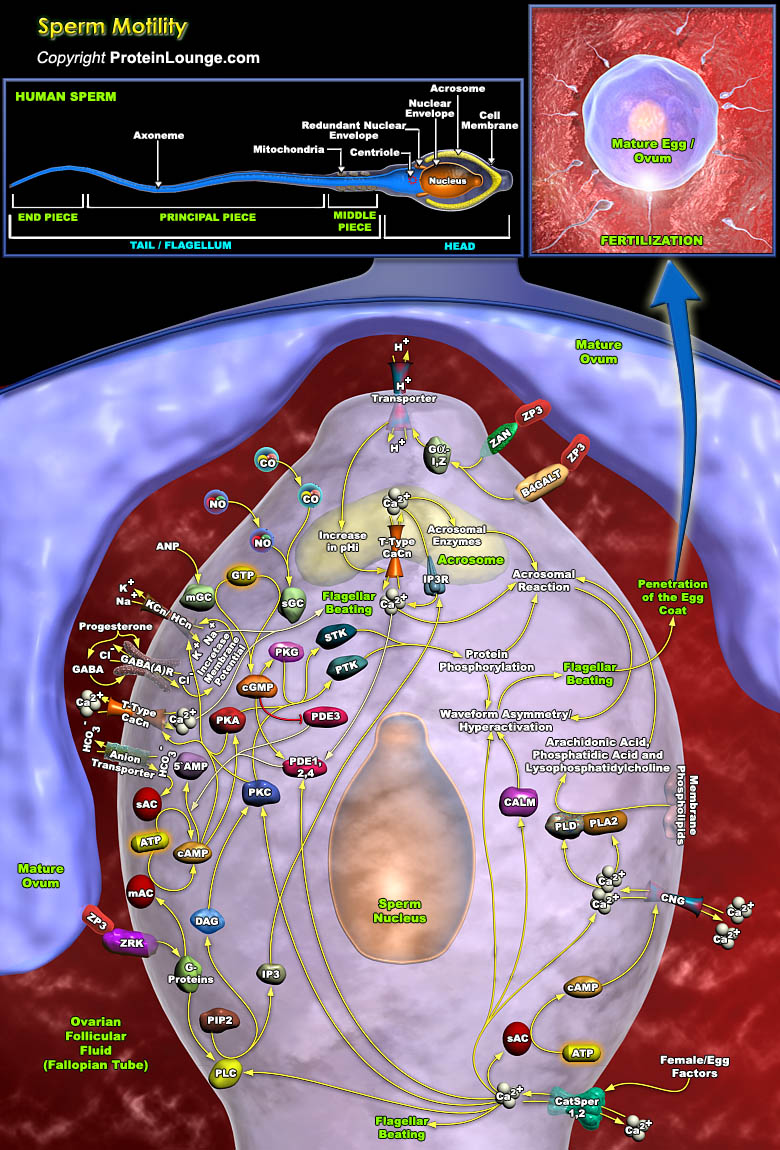
In humans, roughly 300 million spermatozoa on average are ejaculated in the female reproductive tract, but only about one of every million actually enters the Fallopian tube. Upon entry, these spermatozoa apparently bind strongly to the oviductal epithelium in the isthmus, forming a sperm storage site. Human spermatozoon formed in the testes via spermatogenesis is morphologically complete but functionally immature and incapable of fertilizing an egg. Spermatozoa to become fertile must undergo maturation in the epididymis followed by capacitation, and acrosome reaction in the female reproductive system. For reaching the oocyte at the fertilization site, the limited number of capacitated spermatozoa in the isthmus has to swim a long way full of obstacles. This[..]
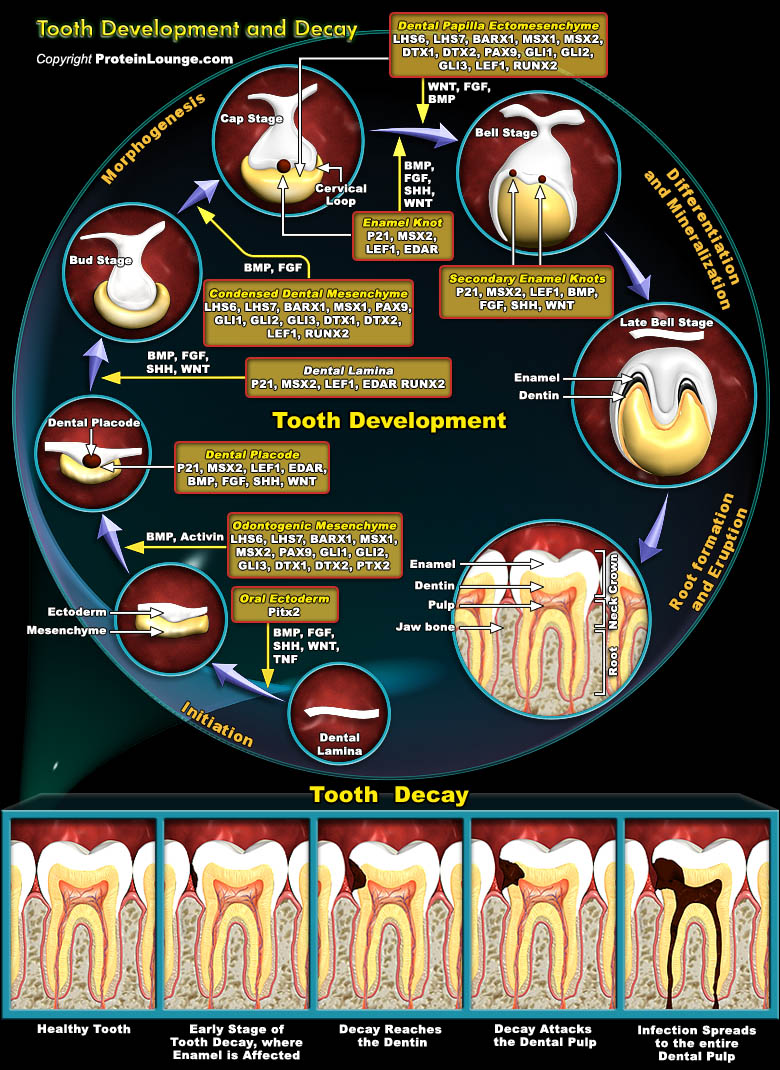
Teeth develop as ectodermal appendages in vertebrate embryos, and their early development resembles morphologically as well as molecularly other organs such as Hairs and Glands. Interactions between the Ectoderm and underlying Mesenchyme constitute a central mechanism regulating the morphogenesis of all these organs. Central features of Tooth morphogenesis are the formation of the Epithelial Placode, the budding of the Epithelium, the condensation of Mesenchyme around the bud, and the folding and growth of the Epithelium generating the shape of the Tooth Crown. The mineralized structures characteristic for Teeth, which is, Dentin and Enamel, are formed by specialized cells, the Odontoblasts and Ameloblasts differentiating from the Mesenchyme and Epithelium,[..]
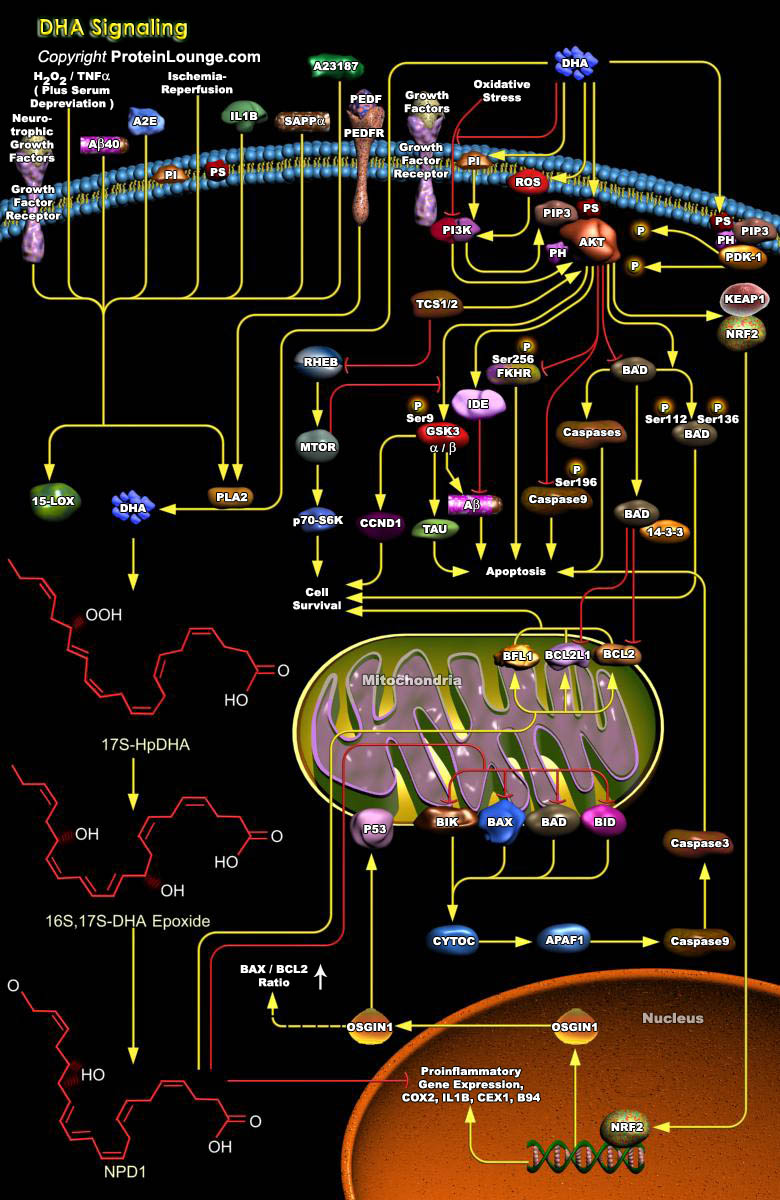
The cell membranes do not simply serve as barriers to separate the inside of the cell from the outside or to delineate different intracellular compartments. These membranes also serve as a platform for cell signaling by allowing specific sets of proteins to interact. Phospholipids are major structural constituents of the cell membranes. In the cell membranes of neurons, the two most prevalent Phospholipids include PC (Phosphatidylcholine) and PS (Phosphatidylserine). When cell membranes are stimulated by cell signaling activity, enzymes (called Phospholipases) free lipid messengers from these reservoirs. The lipid messengers then regulate and interact with other signaling cascades to contribute to the development, differentiation, function protection, and repair of the[..]
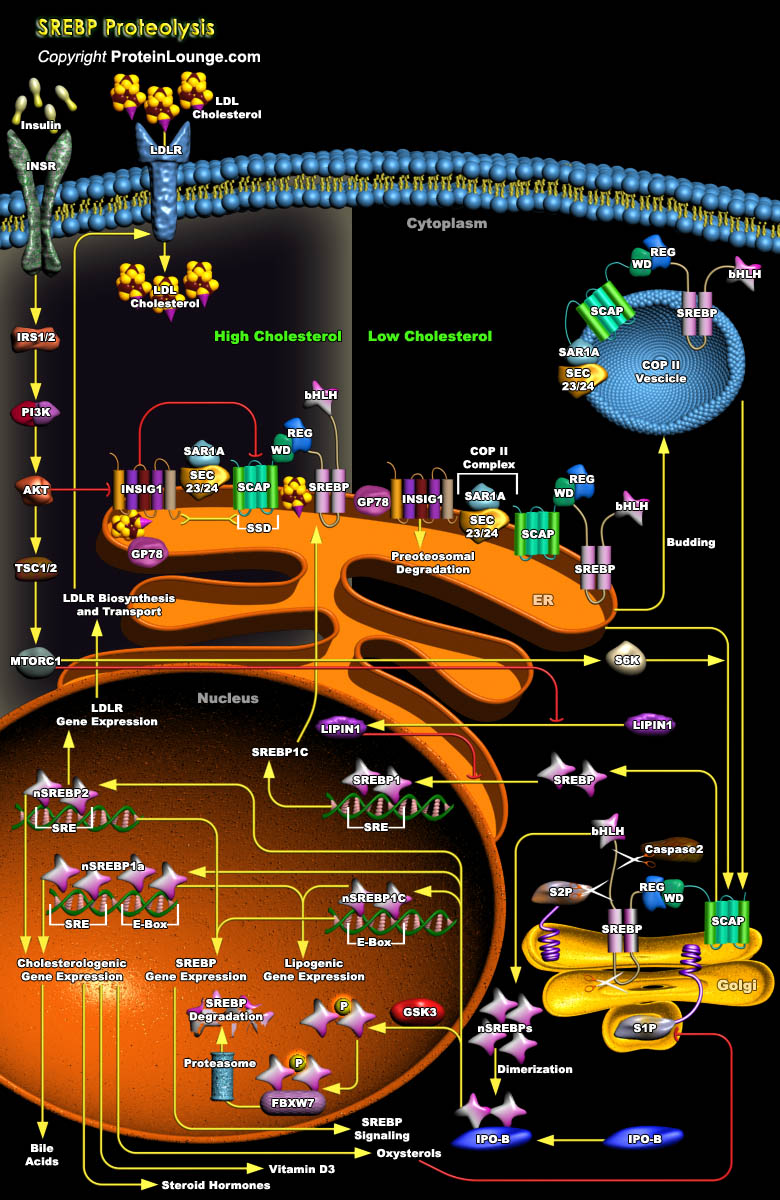
Cellular Lipid homeostasis in mammalian cells is regulated through the end-product feedback regulation of Lipid synthesis by a family of membrane-bound transcription factors designated SREBPs (Sterol Regulatory Element–Binding Proteins) that control the flux of cellular metabolites into the major Lipid pathways. The mammalian cell continuously adjusts its Sterol content by regulating levels of key Sterol synthetic enzymes and levels of Lipoprotein receptors that mediate uptake of Cholesterol-laden particles. Control is brought about by SREBPs, which monitor the Sterol-regulated transcription and directly activate the expression of more than 30 relevant genes dedicated to the synthesis and uptake of Cholesterol, Fatty Acids, Triglycerides, and Phospholipids, as well[..]
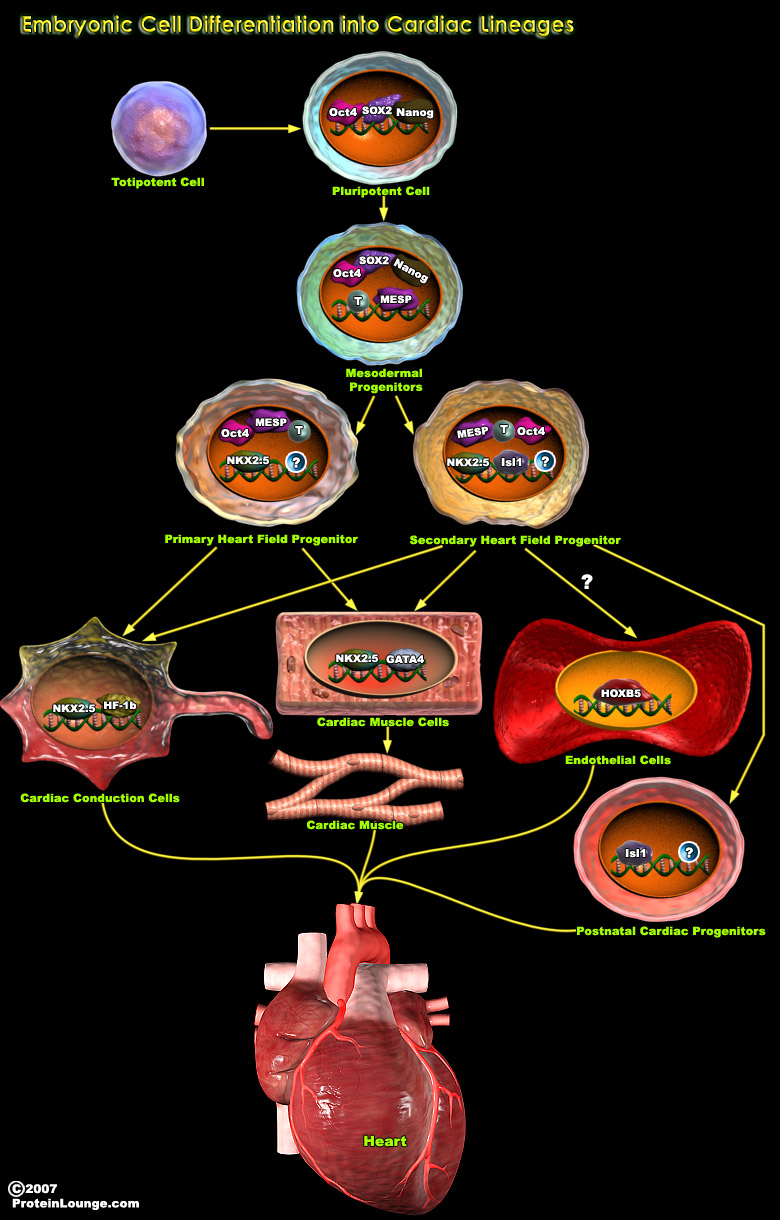
MI(Myocardial infarction ) causes the loss of cardiac tissue and scar formation, which ultimately lead to heart failure. According to the World Health Organization, heart failure initiated by MI and coronary artery disease accounts for 29% of deaths worldwide. However, human heart tissue does not regenerate spontaneously, thus “regenerative medicine” represents a promising alternative treatment for MI. Cardiac tissue regenerative medicine involves cardiomyocyte regeneration, neovascularization, and paracrine cytokines, which have anti-inflammatory, anti-apoptotic, and anti-remodeling effects. During the last decade, stem cells have become promising candidates for regenerative medicine not only because of their capacity of differentiation toward[..]
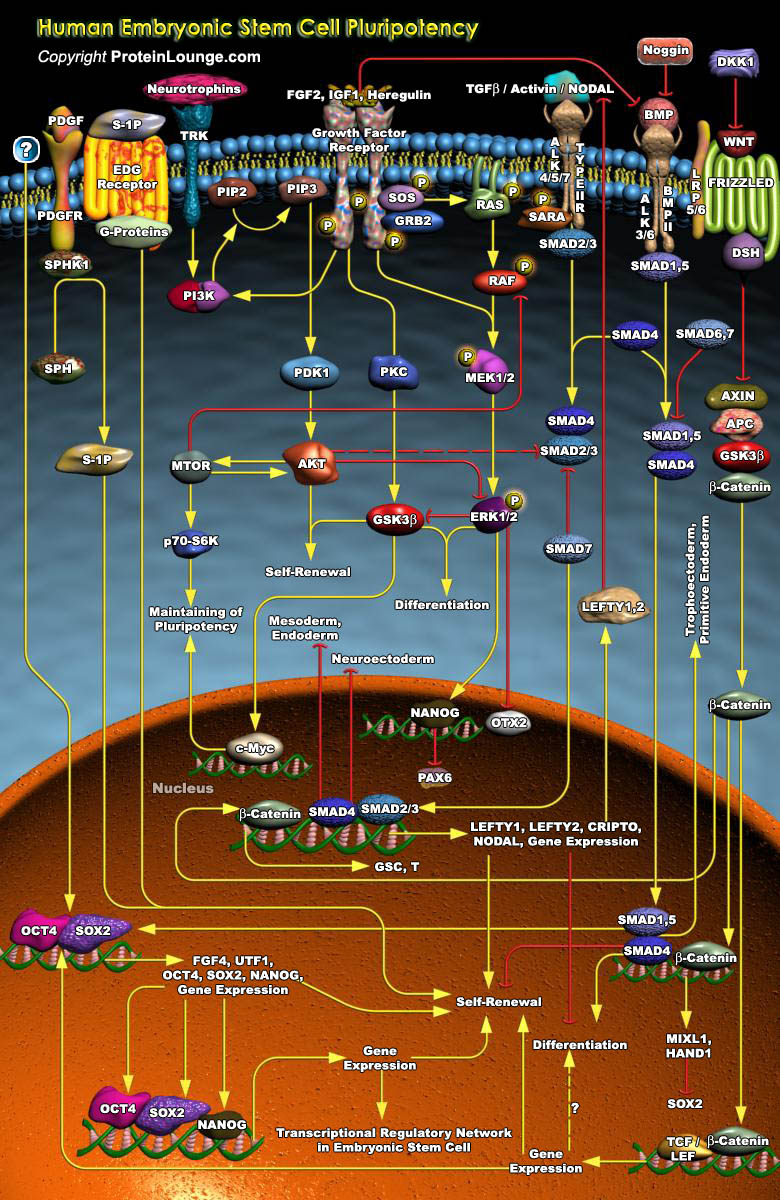
ESCs (Embryonic Stem Cells) are Pluripotent cells capable of differentiating into any cell type of the body. Only three species of Mammals have yielded long-term cultures of self-renewing ESCs- Mice, Monkeys, and Humans. Human ESCs are derived from Blastocysts, multicellular structures originating from four cleavages of fertilized oocytes. Isolated from the ICM (Inner Cell Mass) of Blastocysts, the ESCs retain properties of self-renewal and the potential to be committed and to differentiate toward most cell lineages. They are able to spontaneously give rise to different progenies of the three embryonic layers, namely, the Ectoderm, the Mesoderm and the Endoderm. The Pluripotency of ESCs has attracted great attention for their potential use in tissue and cell therapy.[..]

