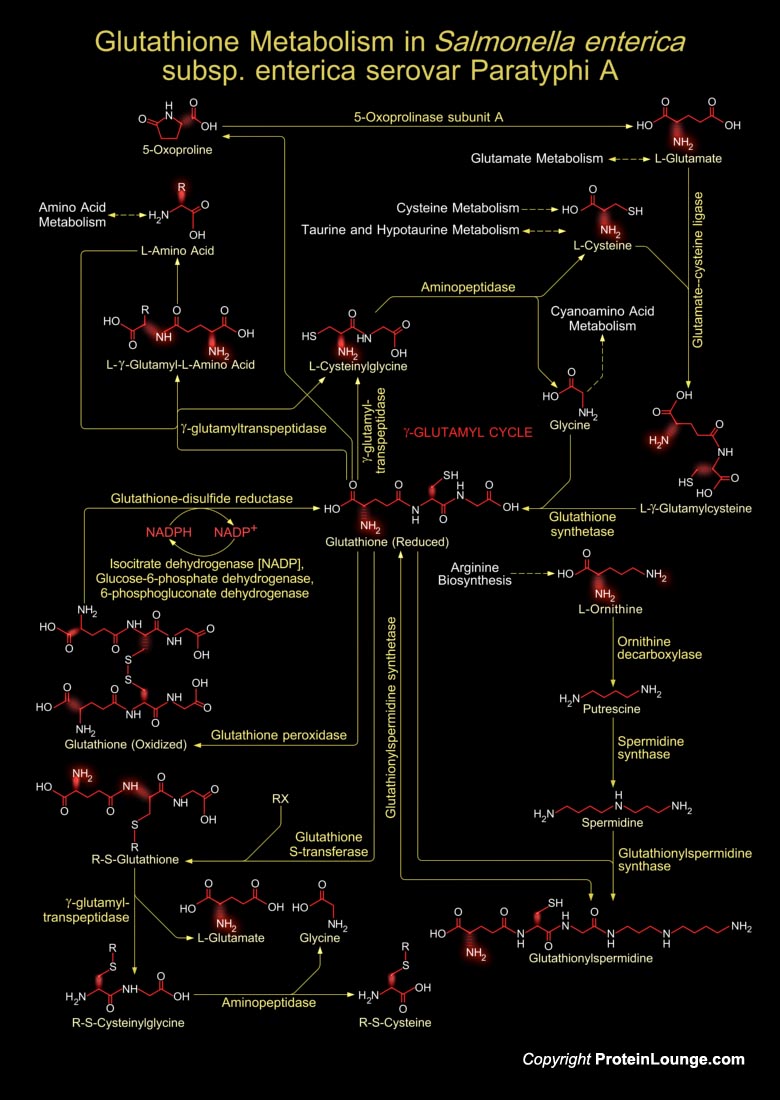
Salmonella enterica subsp. enterica is a subspecies of Salmonella enterica, the rod-shaped, flagellated, aerobic, Gram-negative bacterium. Many of the pathogenic serovars of the S. enterica species are in this subspecies, including that responsible for typhoid. Salmonella is classified into >2500 serovars. Among the >2500 Salmonella serovars, several serovars have been identified as major pathogens to humans and domestic animals, including Salmonella Typhimurium, Enteritidis, Typhi, Newport, Heidelberg and Paratyphi A (Ref.1). Six subspecies of S. enterica are currently recognized in Salmonella enterica. Subspecies I (subspecies enterica) is responsible for nearly all infections in humans and warm-blooded animals, while five other subspecies are isolated[..]
Glutathione is a sulfhydryl (-SH) antioxidant, antitoxin, and enzyme cofactor. It is ubiquitous in animals, plants, and microorganisms, and being water soluble is found mainly in the cell cytosol and other aqueous phases of the living system. It cannot enter most cells directly and therefore must be made available inside the cell from its three constituent amino acids: Glycine, Glutamate and Cysteine. The rate at which glutathione can be made depends on the availability of Cysteine, which is relatively scarce in foodstuffs. Furthermore, the Cysteine molecule has a sulfur-containing portion which gives the whole Glutathione molecule its ‘biochemical activity’. Cysteine can also enter the Glutathione metabolism through several other metabolic pathways like[..]
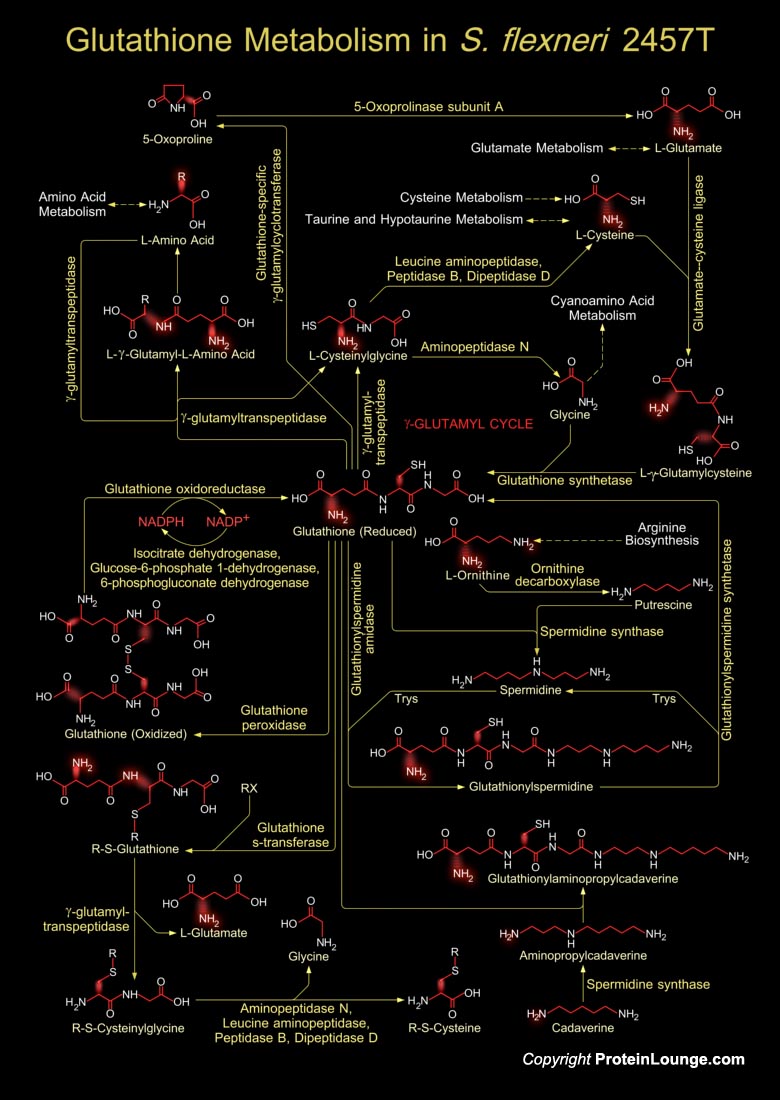
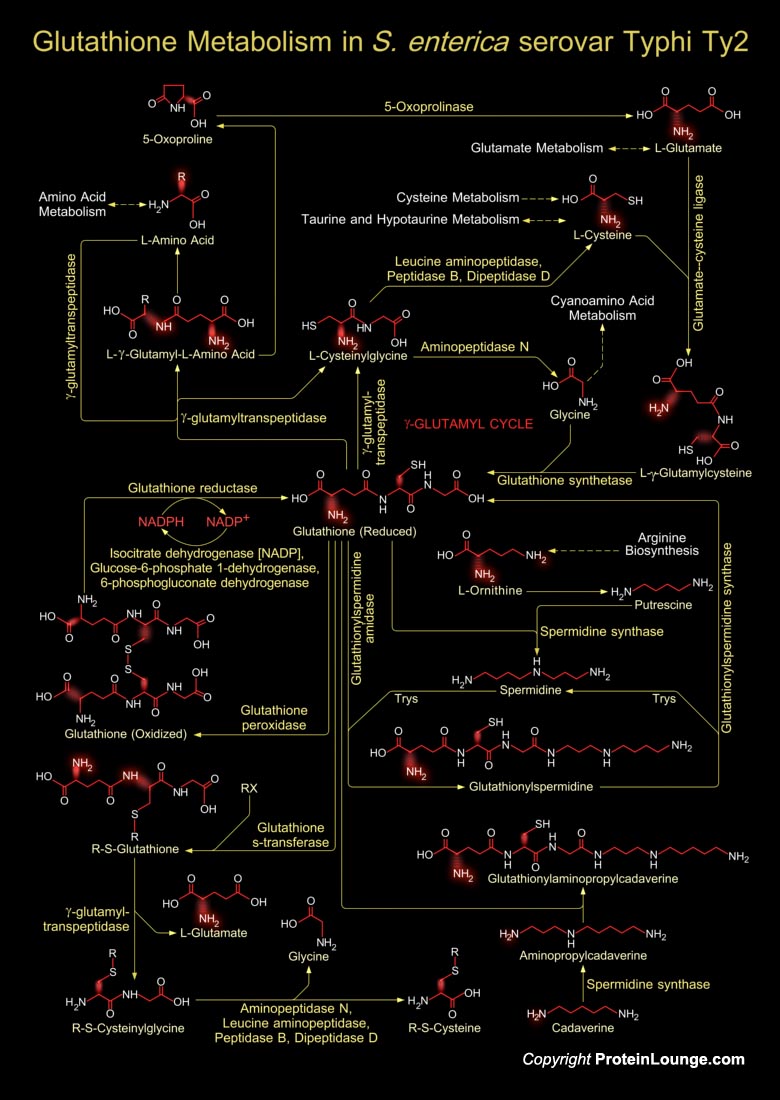
Shigella is a Gram-negative, non-sporulating, facultative anaerobic bacterium that causes Dysentery or Shigellosis in man. Shigella is highly invasive in the colon and the rectum, and is able to proliferate in the host cell cytoplasm, triggering an inflammatory reaction. The strain S. flexneri 2457T harbors four plasmids, which remains to be completed. Glutathione metabolism in Shigella occurs within cells in two closely linked, enzymatically controlled reactions that utilize ATP and draw on nonessential amino acids as substrates. Glutathione is a tripeptide, composed of glutamate, cysteine and glycine, and has numerous important functions within the bacterial cell. This tripeptide is specifically a thiol compound, present in the highest concentration in all types of[..]
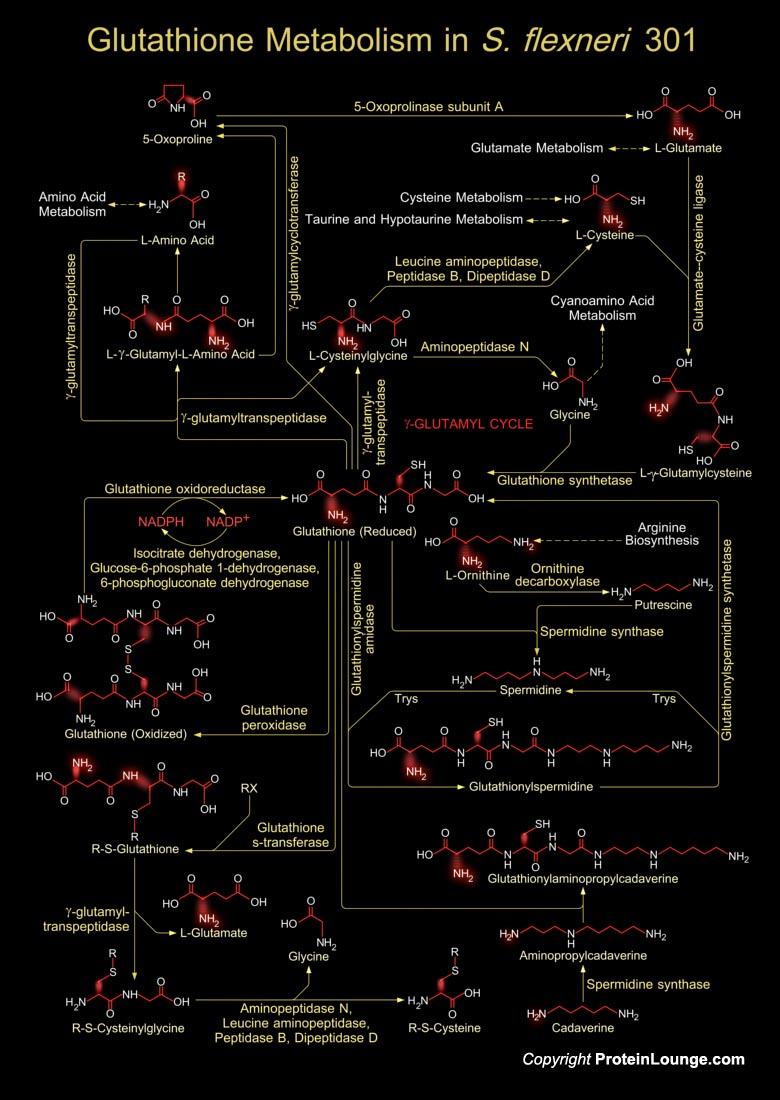
Shigella is a Gram-negative, non-sporulating, facultative anaerobic bacterium that causes Dysentery or Shigellosis in man. Shigella is highly invasive in the colon and the rectum, and is able to proliferate in the host cell cytoplasm, triggering an inflammatory reaction. S. flexneri 2a strain, 301 has been recently sequenced (Ref.1). Glutathione metabolism in Shigella occurs within cells in two closely linked, enzymatically controlled reactions that utilize ATP and draw on nonessential amino acids as substrates. Glutathione is a tripeptide, composed of glutamate, cysteine and glycine, and has numerous important functions within the bacterial cell. This tripeptide is specifically a thiol compound, present in the highest concentration in all types of cells (Ref.2).During[..]
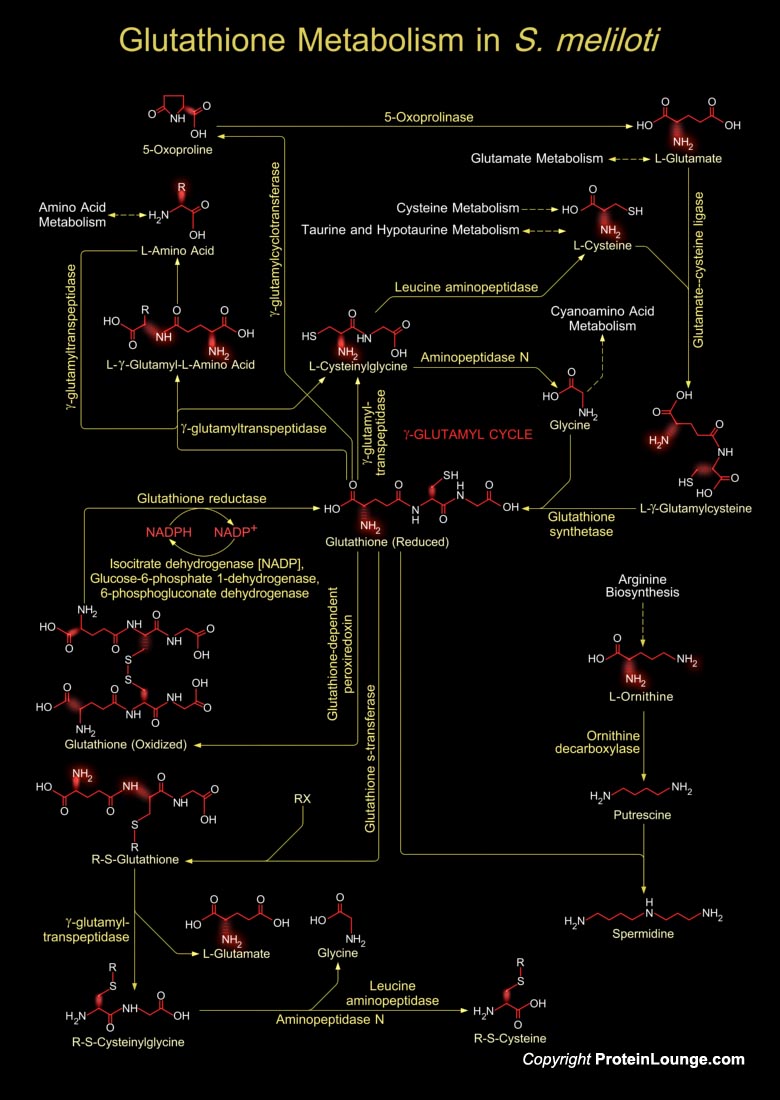
Glutathione is a sulfhydryl (-SH) antioxidant, antitoxin, and enzyme cofactor. It is ubiquitous in animals, plants, and microorganisms, and being water soluble is found mainly in the cell cytosol and other aqueous phases of the living system. Glutathione is a tripeptide composed of Glutamate, Cysteine and Glycine that has numerous important functions within cells. Glutathione is homeostatically controlled, both inside the cell and outside. It often attains millimolar levels inside cells, which makes it one of the most highly concentrated intracellular antioxidants. Glutathione exists in two forms. The antioxidant "reduced Glutathione" tripeptide is conventionally called Glutathione and abbreviated Gsh; the oxidized form is a sulfur-sulfur linked compound,[..]
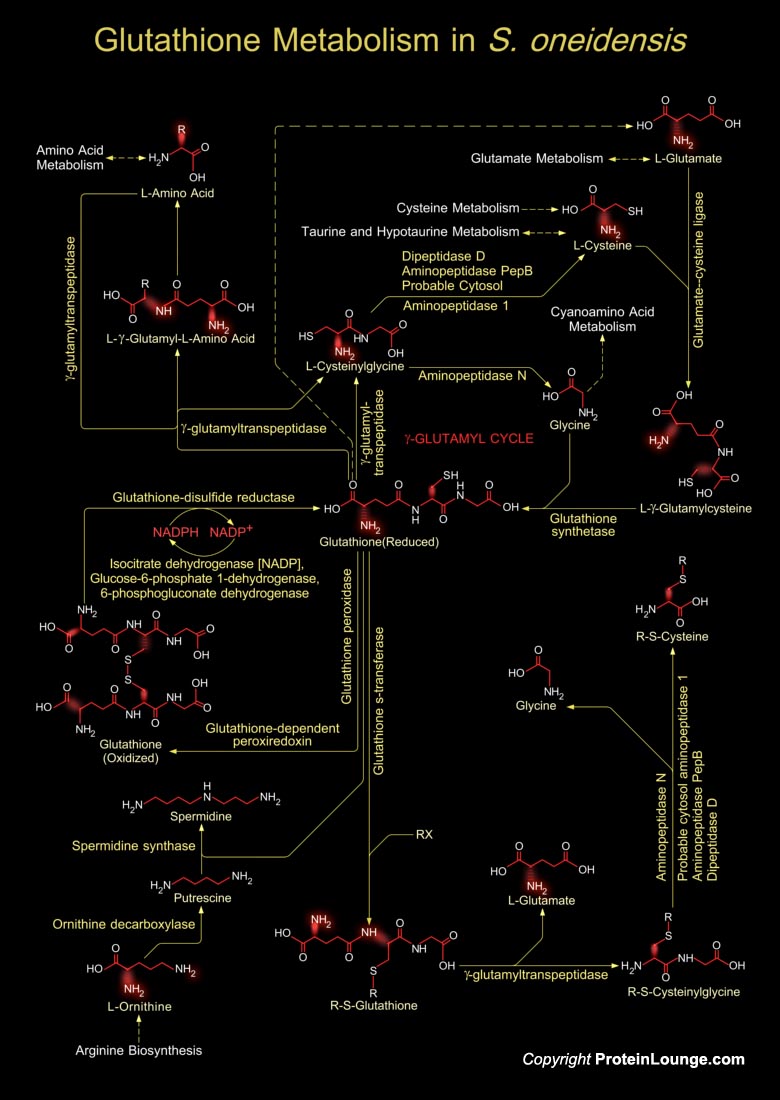
Shewanella oneidensis is a facultative aerobic Gram-negative bacterium. It uses oxygen as the terminal electron acceptor during aerobic respiration, but during anaerobic conditions, S. oneidensis undertakes respiration by reducing alternative terminal electron acceptors such as oxidized metals, fumarate, nitrate etc. The microbe can directly reduce both uranium and chromium from the dissolved liquid state. Such abilities facilitate the removal of dilute metal pollutants in both contained-storage and natural sites. Additionally S. oneidensis immobilizes toxic metals through the formation of insoluble metal sulfides. Thus, the bacterium is an important model organism for bioremediation studies because of its diverse respiratory capabilities, conferred in part by[..]
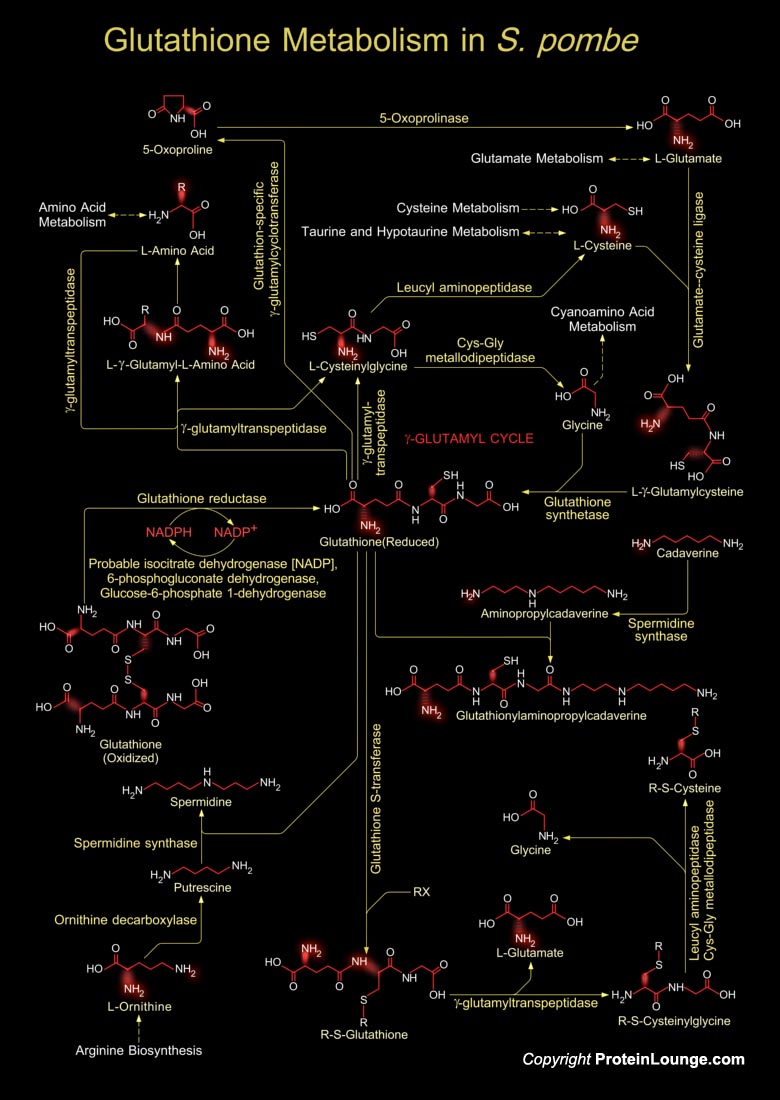
Glutathione is a sulfhydryl (-SH) antioxidant, antitoxin, and enzyme cofactor. It is ubiquitous in animals, plants, and microorganisms, and being water soluble is found mainly in the cell cytosol and other aqueous phases of the living system. It has been assigned several cellular functions, including protection against oxidative damage, maintenance of a reducing cellular thiol-disulfide balance, electron donation for a number of enzymes, protection of protein sulfhydryls from irreversible oxidation, and detoxification of foreign compounds. Glutathione is synthesized enzymatically from its constituent amino acids in two consecutive reactions. GSA1 (Glutathione Synthetase or GSH2) catalyzes the second step and it occurs in two different forms in Schizosaccharomyces[..]
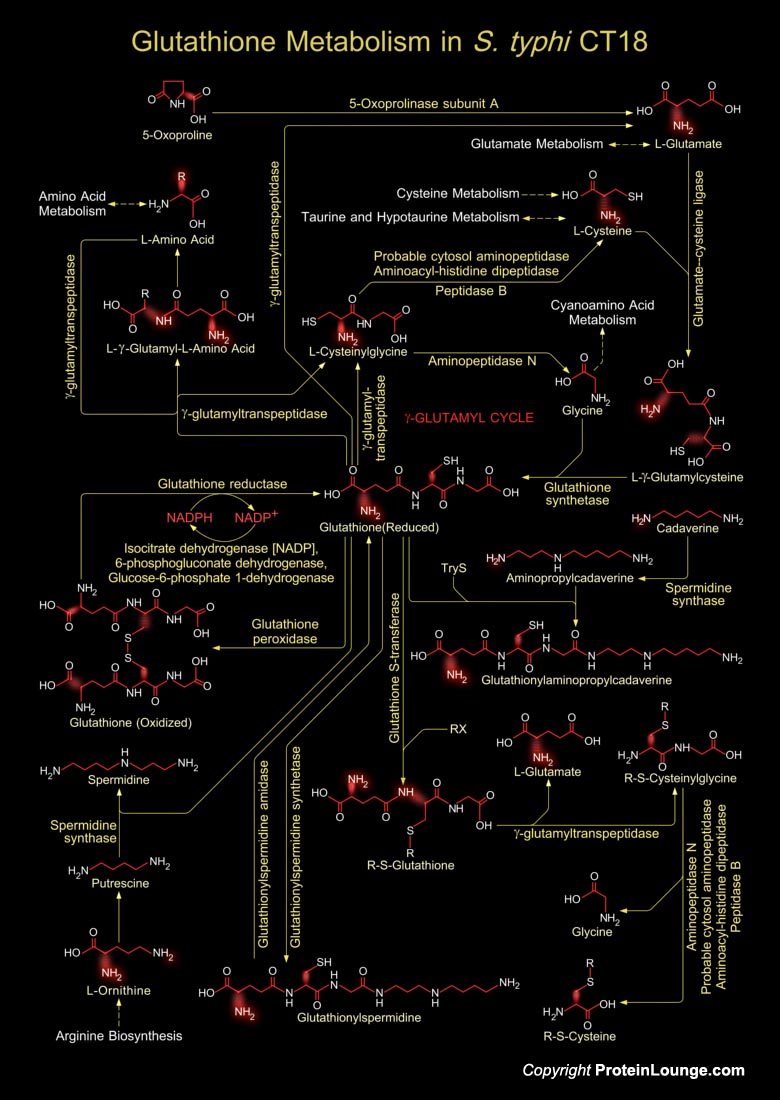
Glutathione is a sulfhydryl (-SH) antioxidant, antitoxin, and enzyme cofactor. It is ubiquitous in animals, plants, and microorganisms, and being water soluble is found mainly in the cell cytosol and other aqueous phases of the living system. It cannot enter most cells directly and therefore must be made available inside the cell from its three constituent amino acids: Glycine, Glutamate and Cysteine. The rate at which glutathione can be made depends on the availability of Cysteine, which is relatively scarce in foodstuffs. It often attains millimolar levels inside cells, which makes it one of the most highly concentrated intracellular antioxidants. Glutathione exists in two forms. The antioxidant "reduced Glutathione" tripeptide is conventionally called[..]
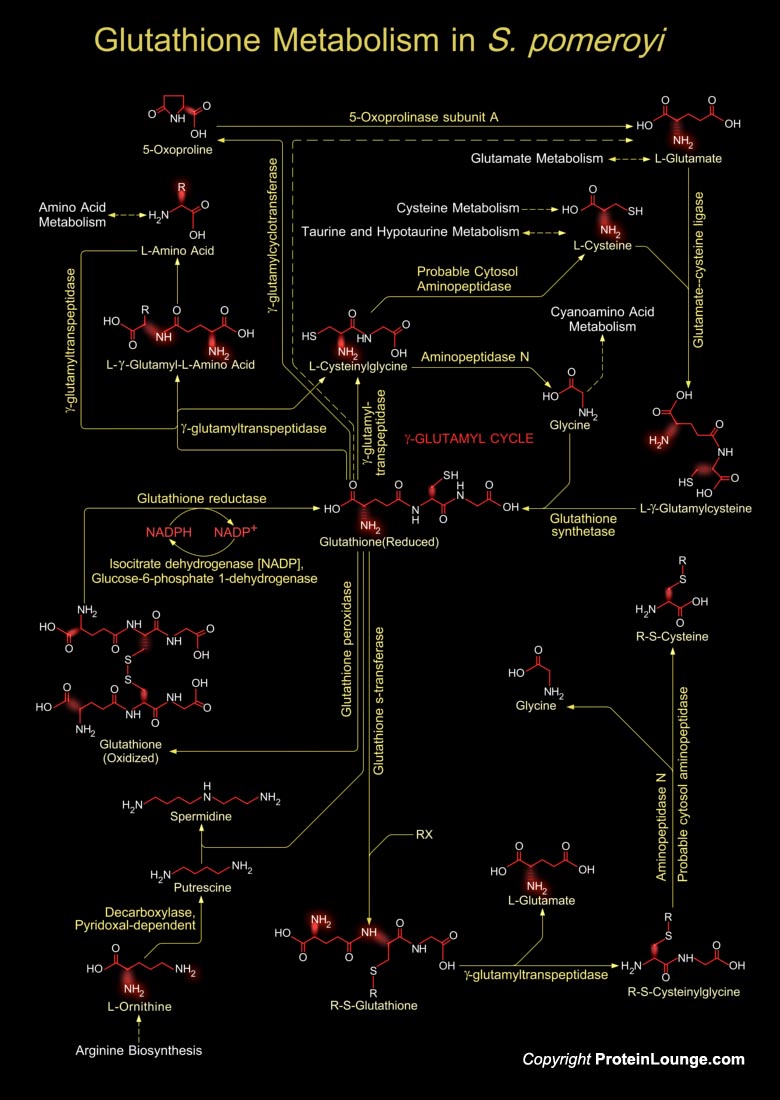
Glutathione is a sulfhydryl (-SH) antioxidant, antitoxin, and enzyme cofactor. It is ubiquitous in animals, plants, and microorganisms, and being water soluble is found mainly in the cell cytosol and other aqueous phases of the living system. Glutathione is a tripeptide composed of Glutamate, Cysteine and Glycine that has numerous important functions within cells. It is homeostatically controlled, both inside the cell and outside and often attains millimolar levels inside cells, which makes it one of the most highly concentrated intracellular antioxidants. Glutathione exists in two forms. The antioxidant "reduced Glutathione" tripeptide is conventionally called Glutathione and abbreviated Gsh; the oxidized form is a sulfur-sulfur linked compound, known as[..]
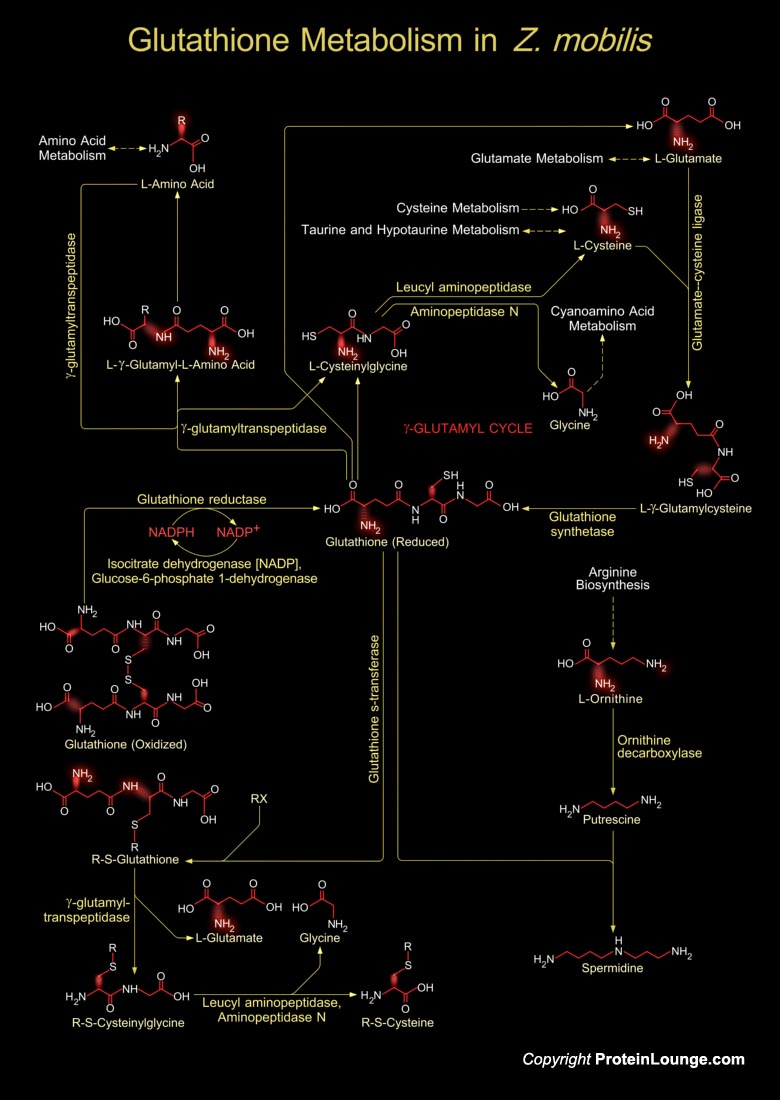
Zymomonas mobilis is an ethanologenic microorganism used for the production of fuel ethanol (Ref.1). Glutathione metabolism in Z. mobilis involves both the synthesis of Glutathione and its catabolism. Glutathione is a small molecule found in almost every cell. It cannot enter most cells directly and therefore must be made available inside the cell from its three constituent amino acids: Glycine, Glutamate and Cysteine. The rate at which Glutathione can be made depends on the availability of Cysteine, which is relatively scarce in foodstuffs. Furthermore, the Cysteine molecule has a sulfur-containing portion which gives the whole Glutathione molecule its ‘biochemical activity’, i.e. its ability to carry out the vitally important functions. Cysteine can also[..]
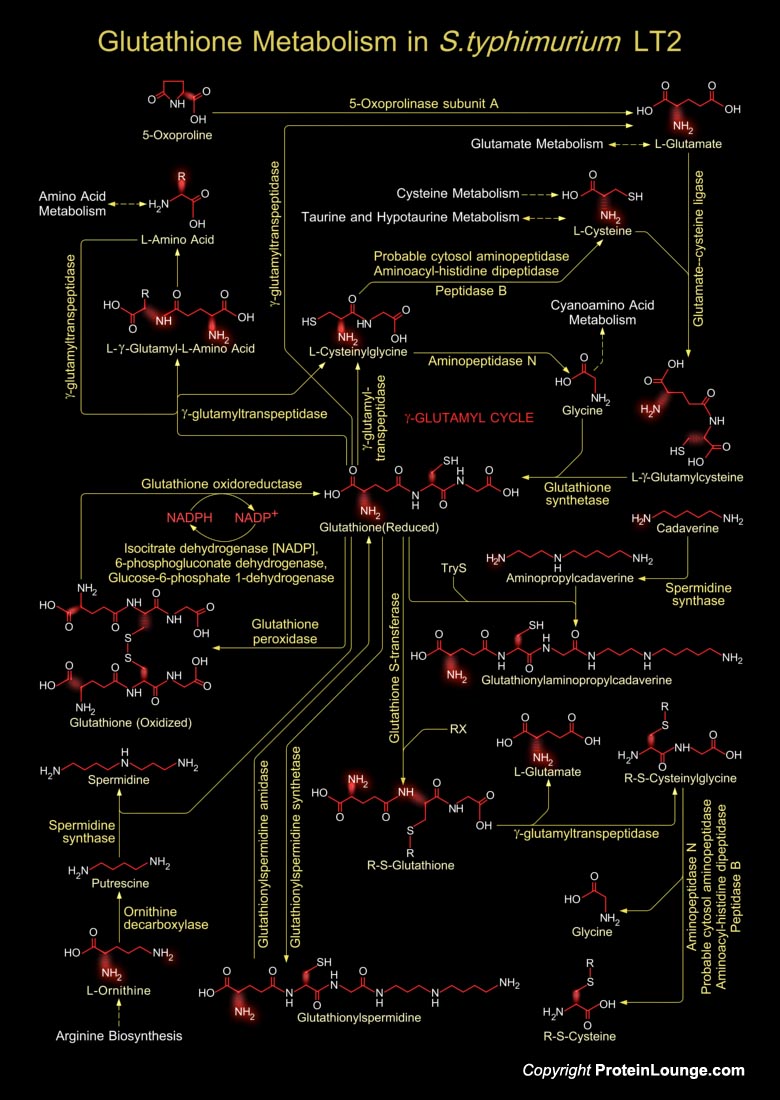
Glutathione is a sulfhydryl (-SH) antioxidant, antitoxin, and enzyme cofactor. It is ubiquitous in animals, plants, and microorganisms, and being water soluble is found mainly in the cell cytosol and other aqueous phases of the living system. It cannot enter most cells directly and therefore must be made available inside the cell from its three constituent amino acids: Glycine, Glutamate and Cysteine. The rate at which glutathione can be made depends on the availability of Cysteine, which is relatively scarce in foodstuffs. Furthermore, the Cysteine molecule has a sulfur-containing portion which gives the whole Glutathione molecule its ‘biochemical activity’. Cysteine can also enter the Glutathione metabolism through several other metabolic pathways like[..]
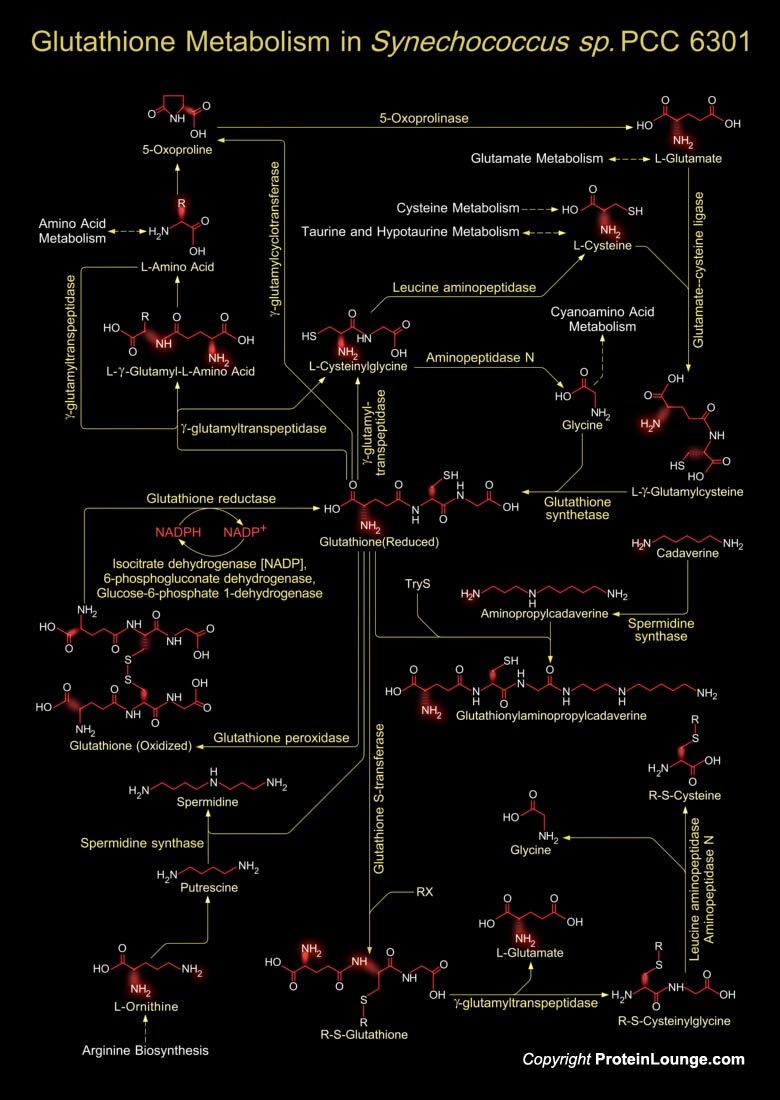
Marine unicellular Cyanobacteria of the Synechococcus group occupy an important position at the base of the marine food web. They are abundant in the world's oceans and as a result are major primary producers on a global scale and one of the most numerous genomes on earth. They have the ability to acquire major nutrients and trace metals at the submicromolar concentrations found in the oligotrophic open seas, and their light-harvesting apparatus is uniquely adapted to the spectral quality of light in the ocean (Ref.1). Glutathione metabolism in Synechococcus sp. involves both the synthesis of Glutathione and its catabolism. Glutathione is a small molecule found in almost every cell. It cannot enter most cells directly and therefore must be made available inside the[..]

