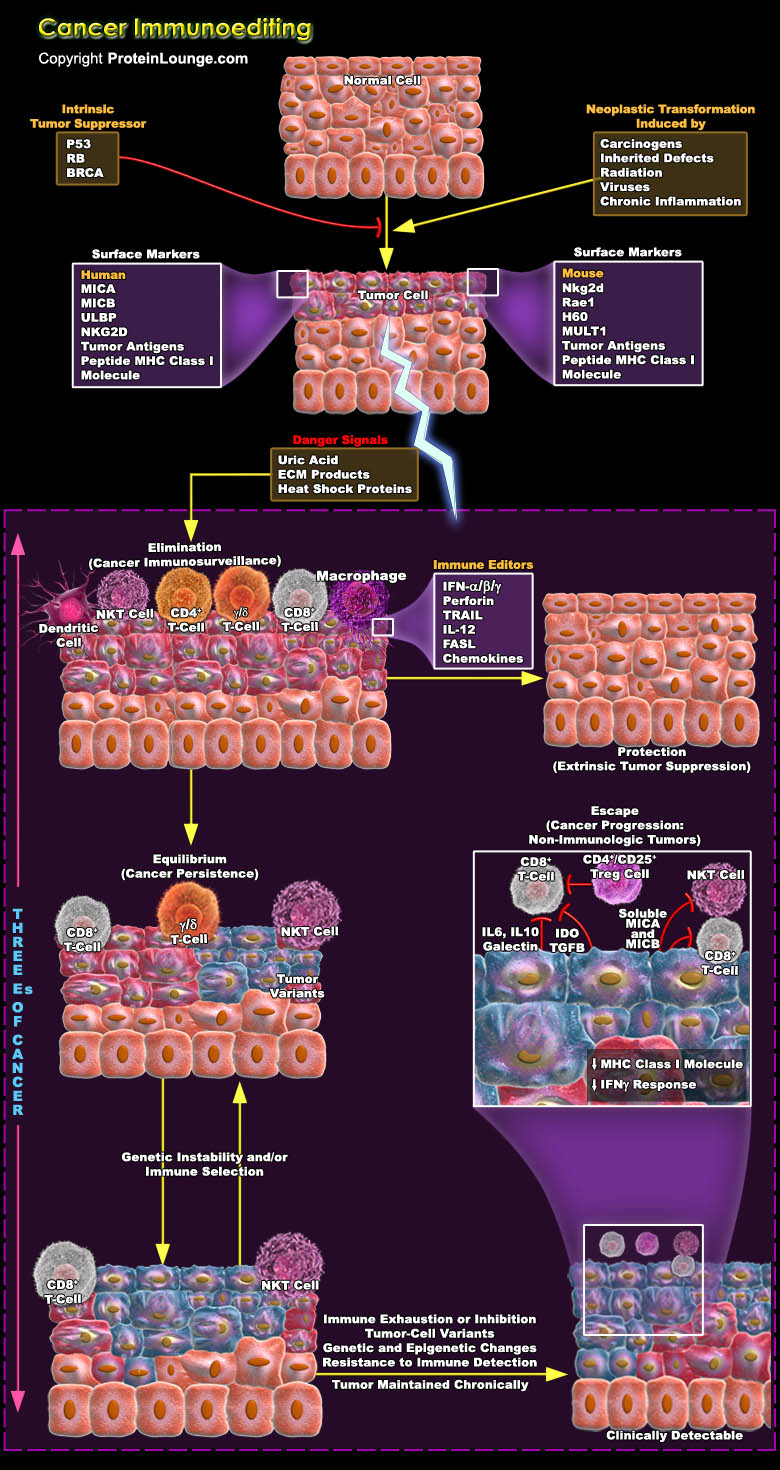
As research into tumour immunology continues at an incredible pace, a considerable amount of work is aimed at exploring the mechanisms that underlie the immunological recognition and elimination of cancer and the downstream consequences of these processes. The capacity of the immune system for recognition is not limited solely to the classic models of self versus pathogen or self versus non-self but encompasses the more-subtle differences that exist between self and transformed self. This conclusion provides the argument for reconsidering the largely discarded hypothesis of cancer immunosurveillance. Immune system attempts to constrain tumour growth, but sometimes tumour cells might escape or attenuate this immune pressure, similar to the way in which these cells[..]
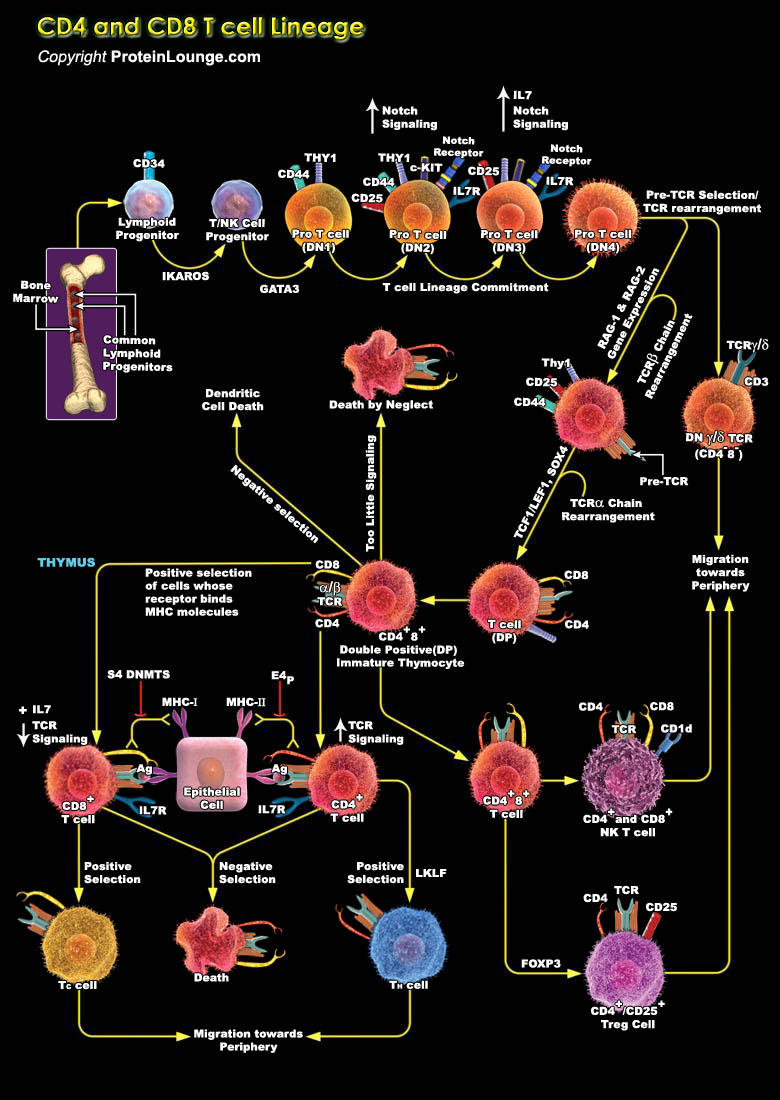
Cell-fate decisions are controlled typically by conserved receptors that interact with co-evolved ligands. Therefore, the lineage-specific differentiation of immature CD4+CD8+ T cells into CD4+ or CD8+ mature T cells is unusual in that it is regulated by clonally expressed, somatically generated T-cell receptors (TCRs) of unpredictable fine specificity. Each mature T cell generally retains expression of the co-receptor molecule (CD4 or CD8) that has an MHC-binding property that matches that of its TCR. Two models were proposed initially to explain this remarkable outcome--'instruction' of lineage choice by initial signalling events or 'selection' after a stochastic fate decision that limits further development to cells with coordinated TCR and co-receptor[..]
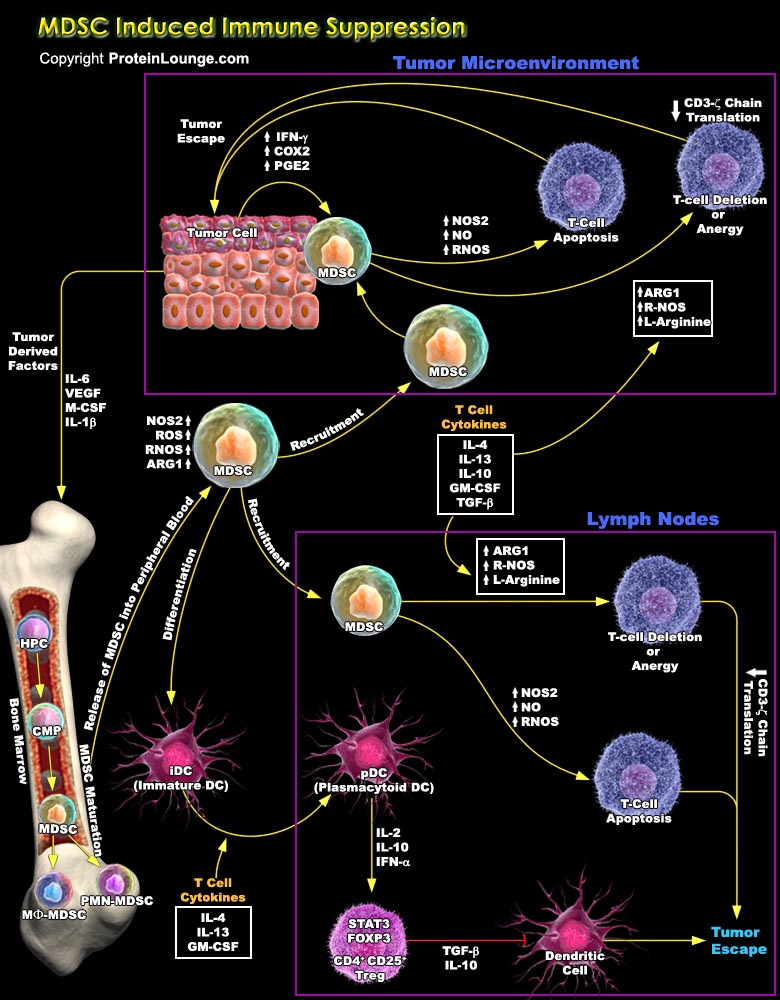
MDSCs (Myeloid-Derived Suppressor Cells) are recently been recognized as critical mediators of tumor progression in numerous solid tumours through their inhibition of tumor-specific immune responses. These cells are increased in numerous pathologic conditions, including infections, inflammatory diseases, graft-versus-host disease, traumatic stress, and neoplastic diseases. MDSCs inhibit not only activation of T cells by anti-CD3 and super antigen, but also antigen-specific CD4+ and CD8+ T-cell responses. The mechanisms of MDSC immunosuppression are diverse, it includes up-regulation of ROS (Reactive Oxygen Species), NO (Nitric Oxide), and L-Arginine metabolism. It also facilitates tumour-induced immune suppression and tumour progression by inducing the[..]
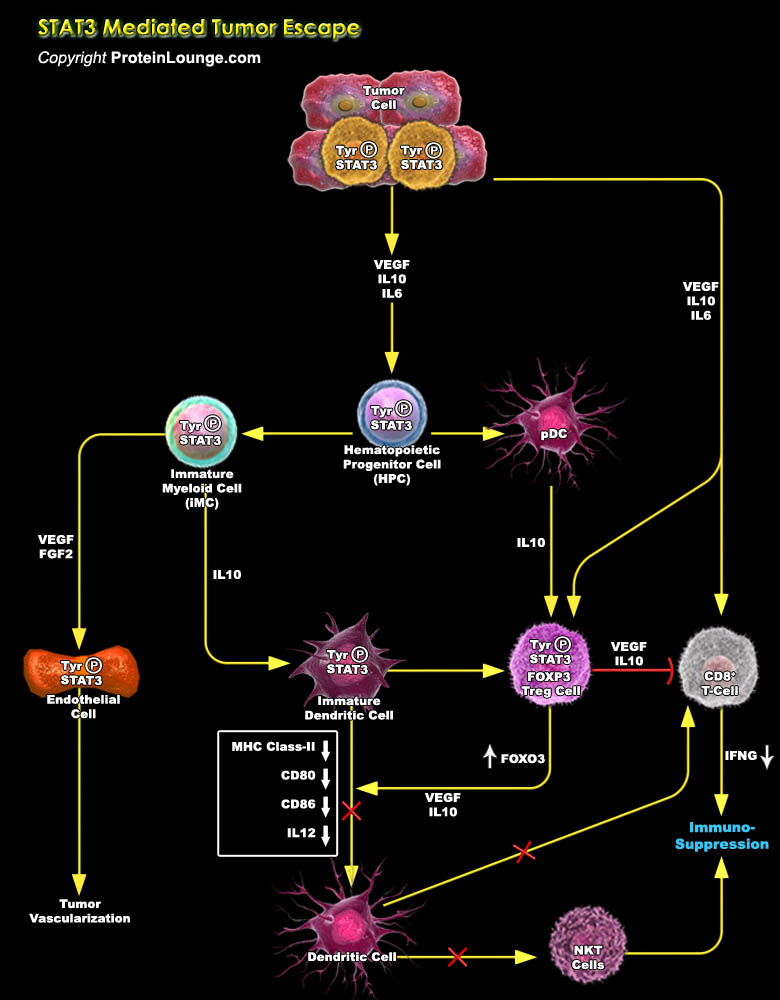
Immune cells in the tumour microenvironment not only fail to mount an effective anti-tumour immune response, but also interact intimately with the transformed cells to promote oncogenesis actively. STAT (Signal Transducer and Activator of Transcription) proteins act as a mediator of cytokine receptor signaling. This protein plays a role in transmitting the signals of growth factor receptors and is both cytoplasmic signaling molecules and nuclear transcription factors that activate diverse genes. In normal cells, STAT activation is transient and tightly regulated. While STAT3 (Signal Transducer and Activator of Transcription 3) acts as a point of convergence for numerous oncogenic signalling pathways, and constitutively remain active both in tumour[..]
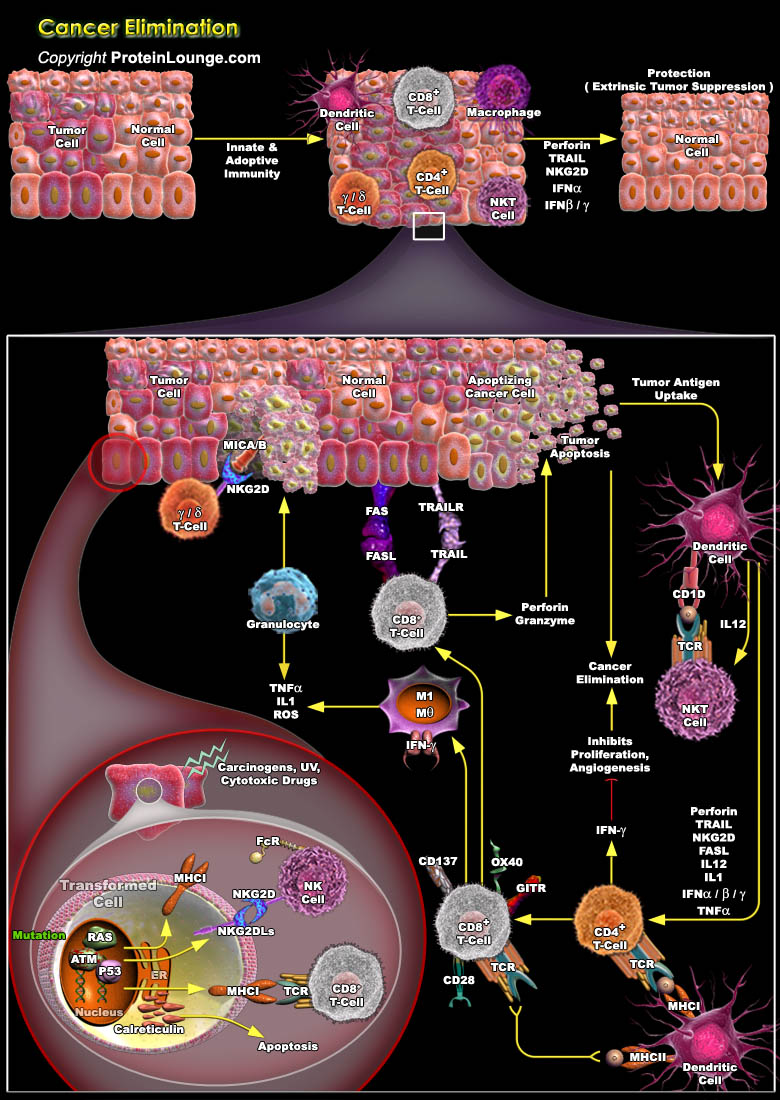
The immune system has three primary roles in the prevention of tumors. First, the immune system can protect the host from virus-induced tumors by eliminating or suppressing viral infections. Second, the timely elimination of pathogens and prompt resolution of inflammation can prevent the establishment of an inflammatory environment conducive to tumorigenesis. Third, the immune system can specifically identify and eliminate tumor cells on the basis of their expression of tumor-specific antigens or molecules induced by cellular stress. The evolution of the cancer immunoediting concept from the older and perhaps more controversial ‘cancer immunosurveillance’ hypothesis has helped interpret the predictive and prognostic significance of immune infiltrates into[..]
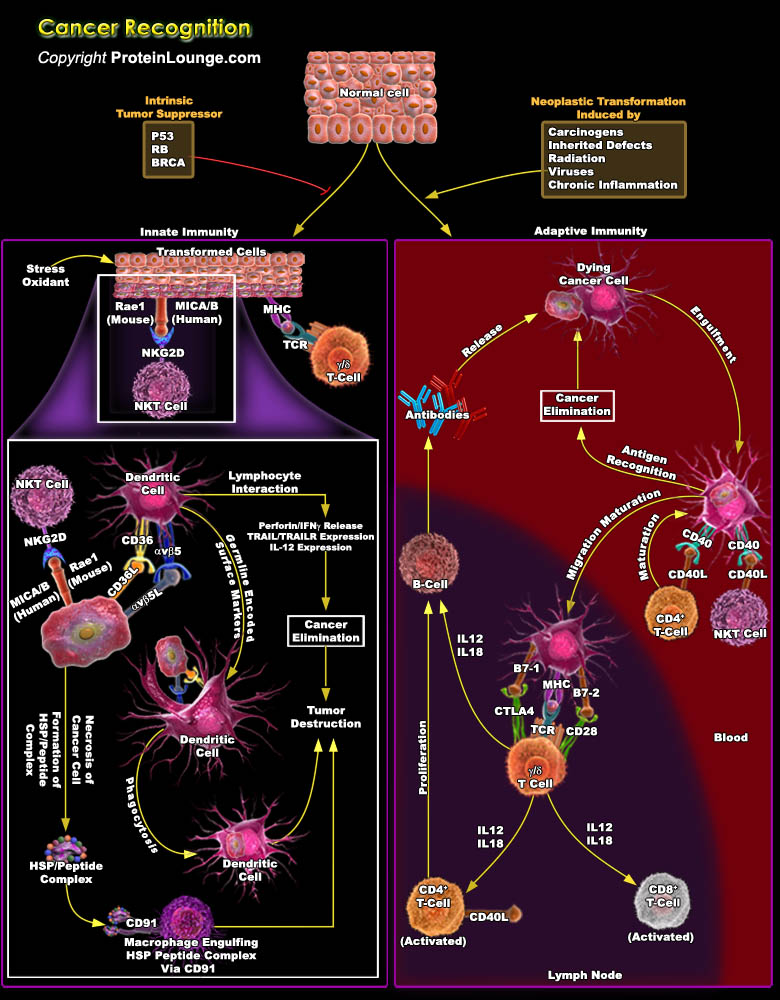
Cancer immunotherapy attempts to exploit the exquisite power and specificity of the immune system for the treatment of malignancy. Although cancer cells are less immunogenic than pathogens, the immune system is clearly capable of recognizing and eliminating tumour cells. However, tumors frequently interfere with the development and function of immune responses. Thus, the challenge for immunotherapy is to use advances in cellular and molecular immunology to develop strategies that effectively and safely augment antitumour responses. By contrast, the immune system has evolved strategies, largely in response to infections, to efficiently search for and specifically destroy diseased targets. Advances in cellular and molecular immunology have provides enormous insights in[..]
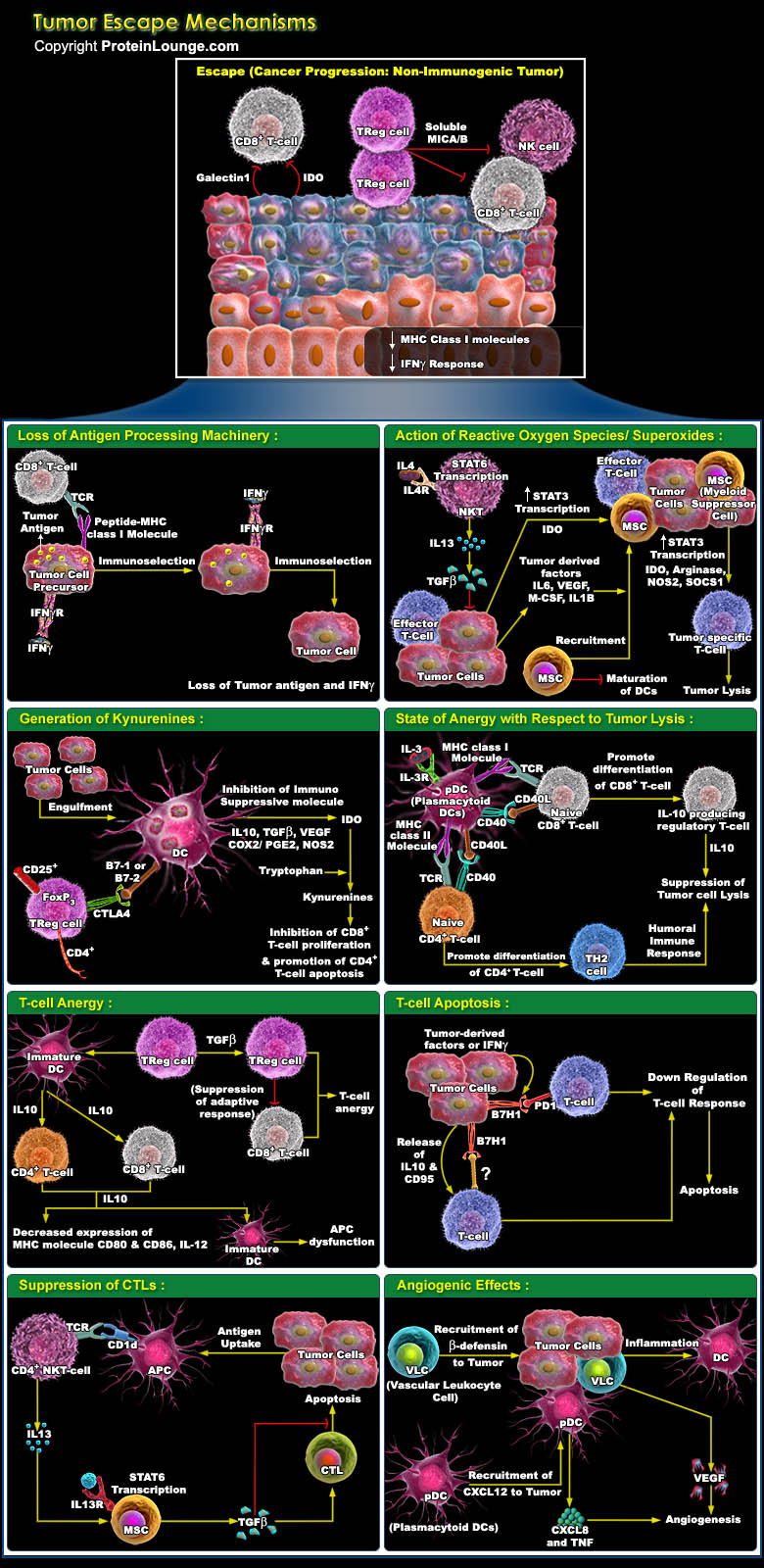
Tumour cells characteristically provides their own growth signals, and ignore growth-inhibitory signals, avoid cell death, replicate without limits, sustain angiogenesis, and invade tissues through basement membranes and capillary walls. Whereas cancer immunosurveillance predicts that the immune system can recognize precursors of cancer and, in most cases, destroy these precursors before they become clinically apparent. But sometimes tumour cells use to escape these innate and adaptive immune responses by immunoselection (that is, selection of non-immunogenic tumour-cell variants, a process that is also known as immunoediting) or by immunosubversion (that is, active suppression of the immune response) and heads towards a difficult situation which is refer as cancer.[..]
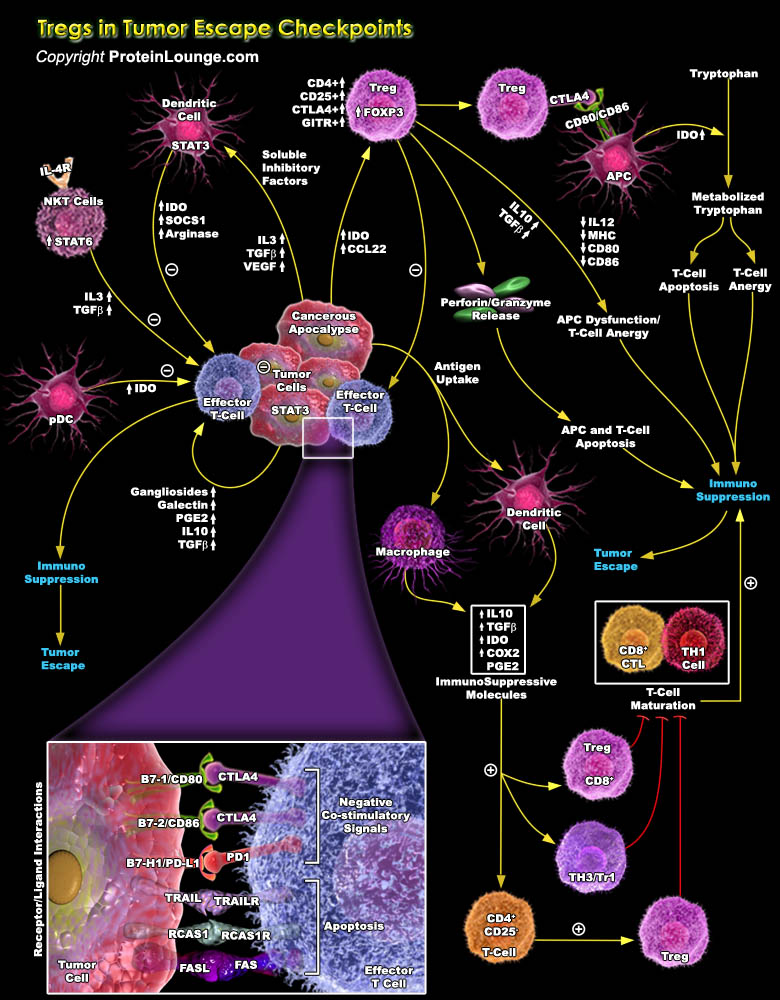
Specific T cell populations have suppressive/regulatory cells known as Regulatory T-cell or Tregs (Previously known as suppressor T-cell). Among them CD4+ regulatory T cells (Tregs) basically has two different subsets Tr1 and Th3 cells which are differentiated by their distinct suppressive mechanisms. The thymus-derived Tregs or natural Tregs (nTregs) express CD4 and high CD25 with FOXP3 (Forkhead Box-P3) to be a key regulatory gene. Apart from these, other markers of Treg include CTLA4 (Cytotoxic T-Lymphocyte-Associated protein-4), GITR (Glucocorticoid-Induced TNFR-Related protein) and LAG-3 (Lymphocyte Activation Gene-3). Treg cells are implicated in the development of autoimmunity, allergy and rejection of organ transplants, as well as the suppression of immune[..]
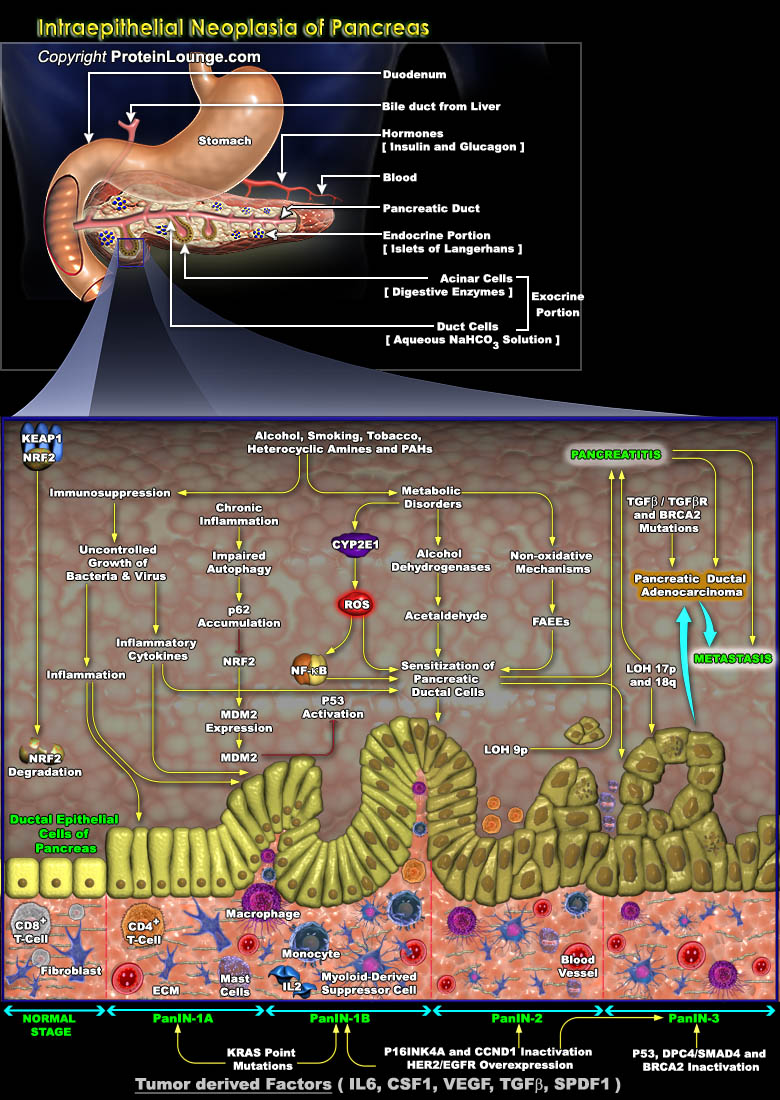
Pancreatic ductal adenocarcinoma is the most common pancreatic neoplasm and as its name suggests it arises from ductal epithelial cells of the pancreas. Other subtypes of pancreatic neoplasms include benign and malignant cystic lesions, mucin producing tumor, acinar cell carcinoma, adenosquamous carcinoma, lymphomas and sarcomas. Pancreatic ductal adenocarcinoma evolves from a progressive cascade of cellular, morphological and architectural changes from normal ductal epithelium through preneoplastic lesions termed PanIN (Pancreatic Intraepithelial Neoplasia). These PanIN lesions are in turn associated with somatic alterations in canonical oncogenes and tumor suppressor genes. The molecular pathogenesis of human pancreatic ductal adenocarcinoma involves the temporal and[..]
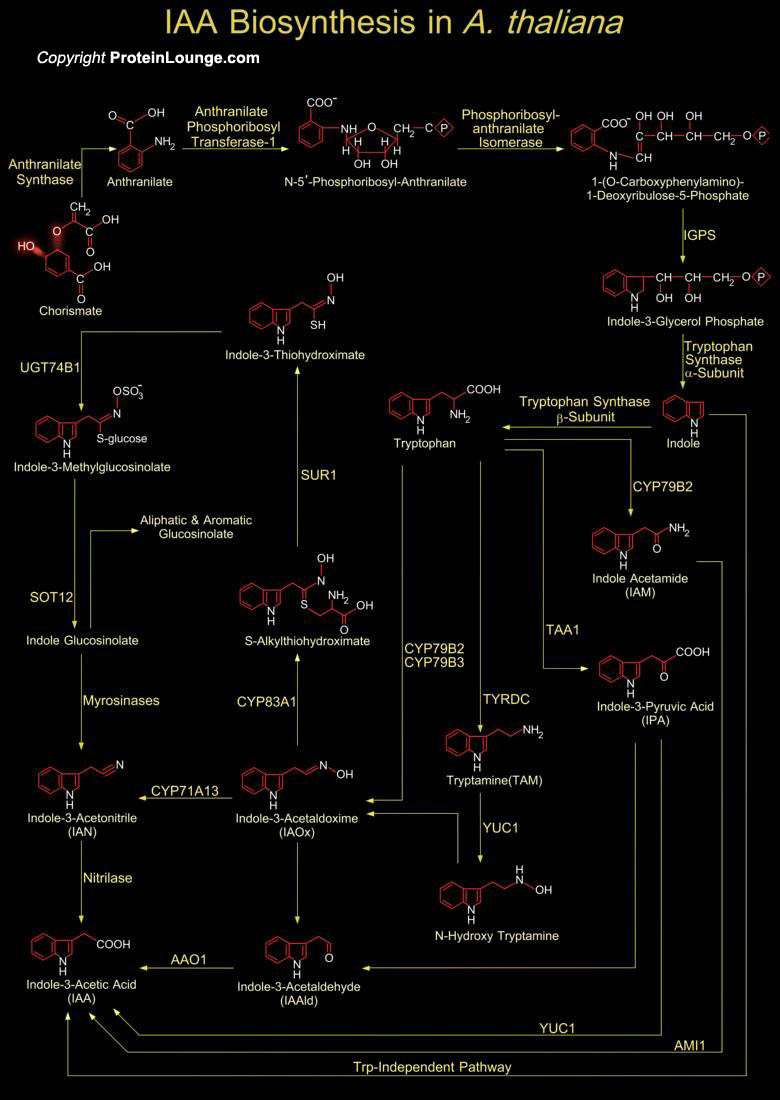
Auxin is a classic Phytohormone involved in a myriad of Developmental and Environmental Processes like Embryo Patterning, Cell Division and Elongation, Vascular Differentiation, Lateral Root Initiation, Gravitropism, and Phototropism. IAA (Indole-3-Acetic Acid) is recognized as the key Auxin in most plants with over 20 members of IAA gene family has been recognized in Arabidopsis. IAA physiologically exists as free acid but IAA can also be found in different conjugated forms, including Ester-types with the Carboxyl group linked via Oxygen to a Sugar (for example Glucose) and Amide-types with the Carboxyl group forming an Amide (Peptide bond) to Amino Acids or Polypeptides. Plants use several pathways to synthesize IAA but none of the pathways is yet defined to[..]
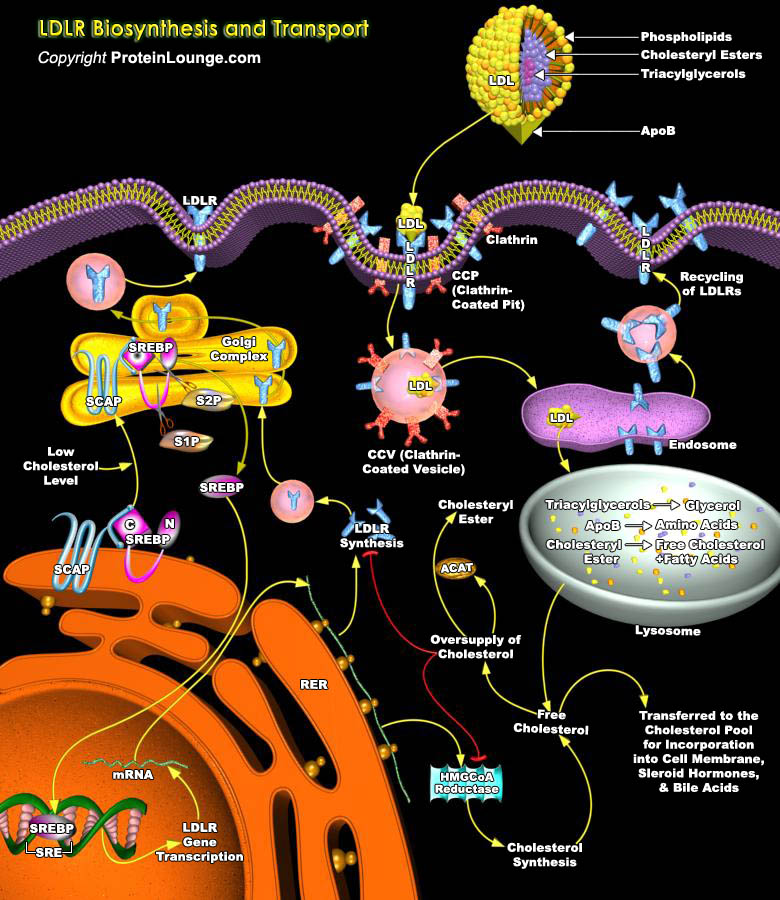
Cholesterol is an important component of cell membrane, and a precursor of Steroid hormones, vitamin D3, and Biliairy Acids. Cell Cholesterol homeostasis is under the control of endogenous Cholesterol synthesis, Cholesterol secretion, and of Lipoprotein Receptor activities that enrich the cell in Cholesterol. Lipoprotein Receptors play an important role in Lipoprotein metabolism and in Cellular Cholesterol homeostasis. LDLs (Low Density Lipoproteins) are the major carriers of Cholesterol as CE (Cholesteryl Esters) in humans. Elevated LDL Cholesterol (>130-160 mg/dl) is one of the major risk factors for CHD (Coronary Heart Disease). For every 1% increase in LDL-Cholesterol, the risk for CHD is increased by 1%-2%. Furthermore, reduction of LDL-Cholesterol[..]
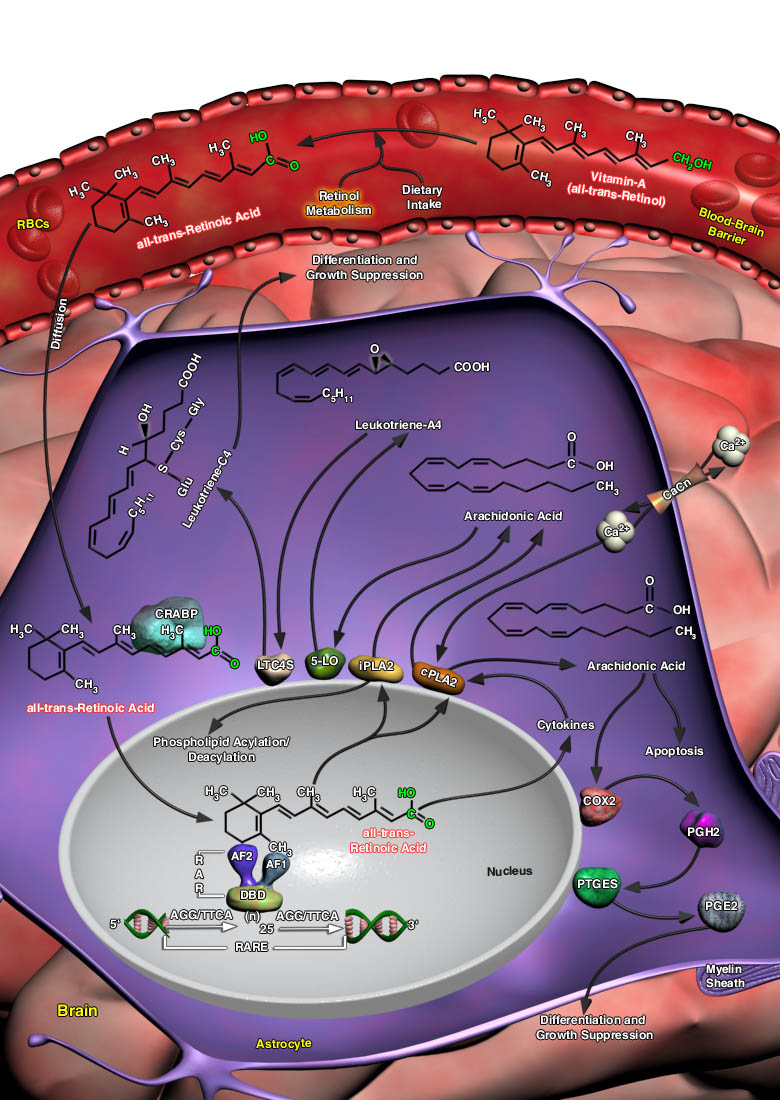
Retinoic Acid, the active form of Vitamin-A (all-trans-Retinol), is a lipophilic molecule, and is known to affect gene transcription. Retinoic Acid is made available in the body through dietary intake and subsequent metabolism in the liver. Vitamin-A is secreted from its storage pools and circulates in blood. In the liver Vitamin-A is converted to all-trans-Retinoic acid, the Carboxylic Acid form of Vitamin-A and diffuses easily to the target tissues through cellular membranes; gets bound to CRABP (Cellular Retinoic Acid Binding Protein) and produces its biological effects through the activation of RARs (Retinoic Acid Receptors). Although biologically active ligands for the RARs also include 9-cis-Retinoic Acid among others, yet circulating levels of 9-cis-Retinoic[..]

