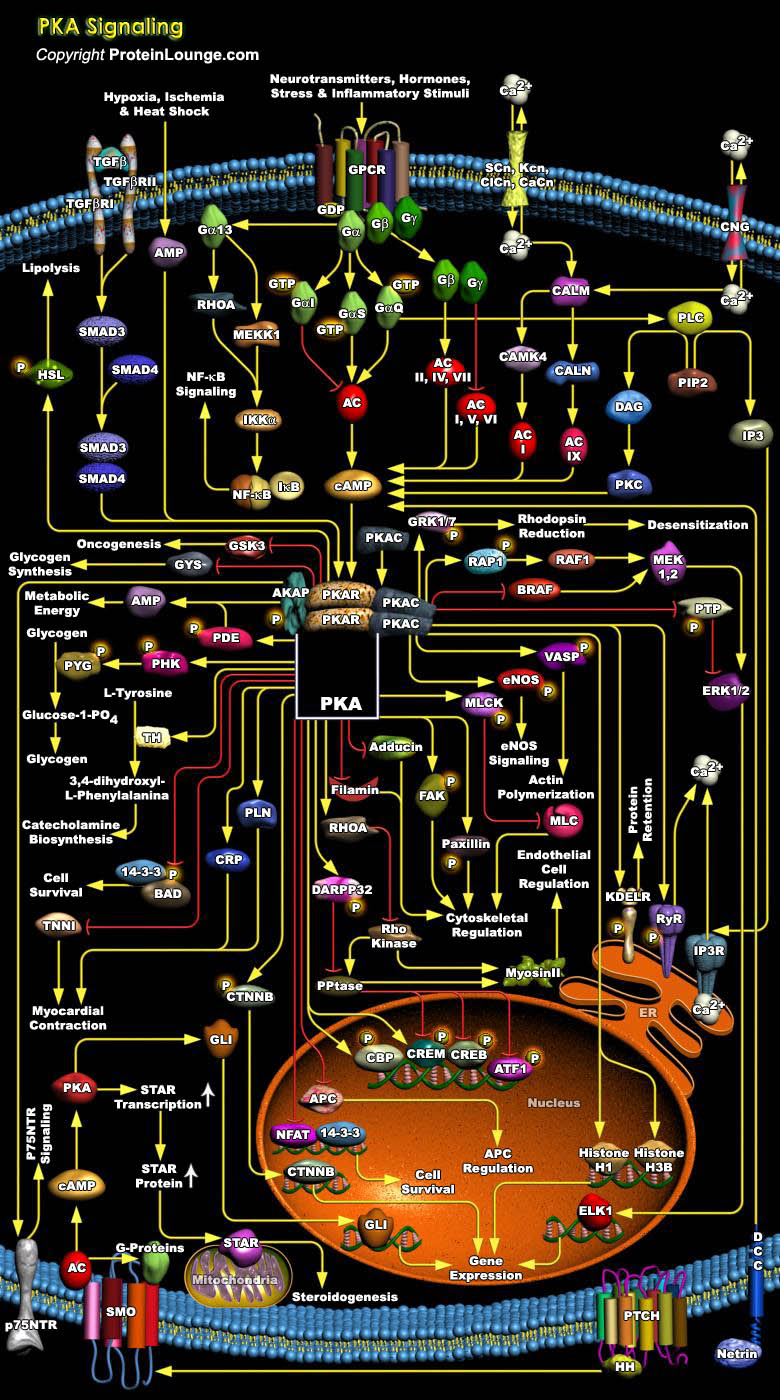
PKA (Protein Kinase-A) is an enzyme that regulates processes as diverse as growth, development, memory, and metabolism. In its inactivated state, PKA exists as a tetrameric complex of two Catalytic subunits (PKA-C) and a Regulatory (PKA-R) subunit dimer. To date, four regulatory subunits have been identified (RI-Alpha, RI-Beta, RII-Alpha and RII-Beta), which are differentially distributed in mammalian tissues. RI-Alpha and RII-Alpha are expressed ubiquitously, RI-Beta is expressed predominantly in the brain and RII-Beta is expressed primarily in brain, adrenal and adipose tissues. These R subunits define Types-I and II PKA, with both types of holoenzyme having three potential C subunits (Alpha, Beta and Gamma). The C-Alpha and Beta subunits share 93% homology and[..]
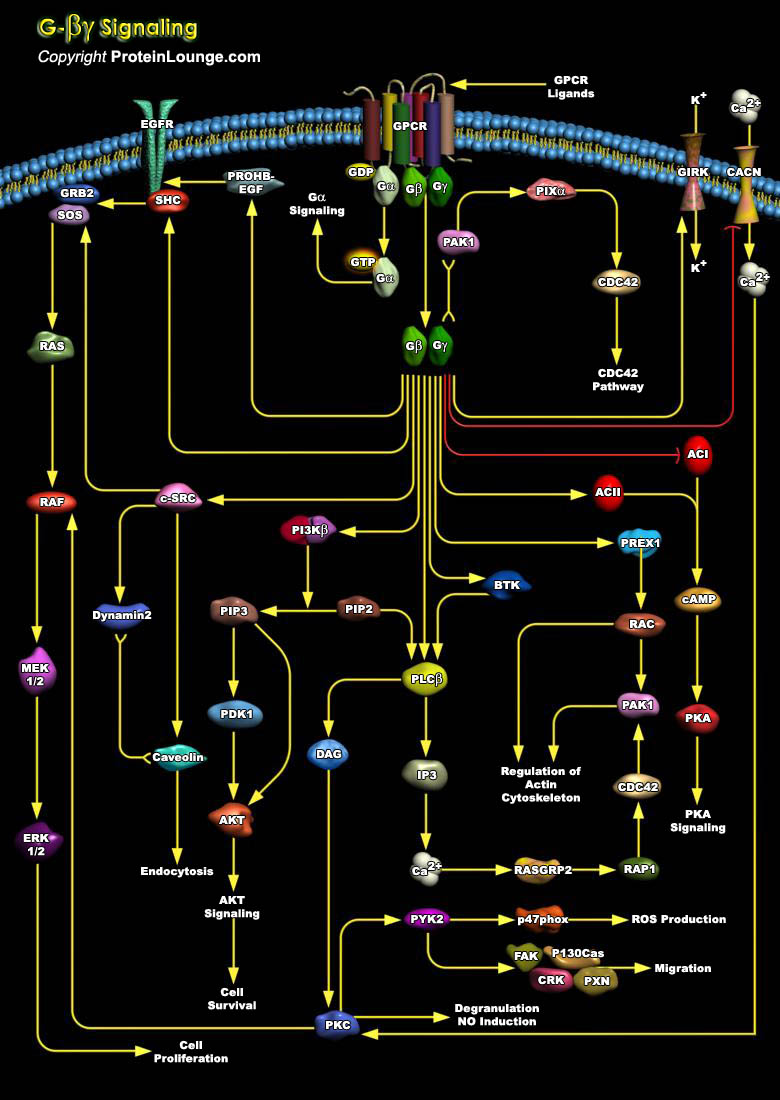
GPCR (G-Protein-Coupled Receptors) constitute a large and diverse family of proteins whose primary function is to transduce extracellular stimuli into intracellular signals. They are among the largest and most diverse protein families in mammalian genomes. Also termed Serpentine receptors, GPCRs are polytopic membrane proteins that share a common structure with seven transmembrane segments, but sequence similarity is minimal among the most distant GPCRs. GPCRs recognize a variety of ligands and stimuli including Peptide and non-peptide Hormones and Neurotransmitters, Chemokines, Prostanoids and Proteinases, Biogenic amines, Nucleosides, Lipids, Growth factors, Odorant molecules and Light. These receptors affect the generation of small molecules that act as[..]
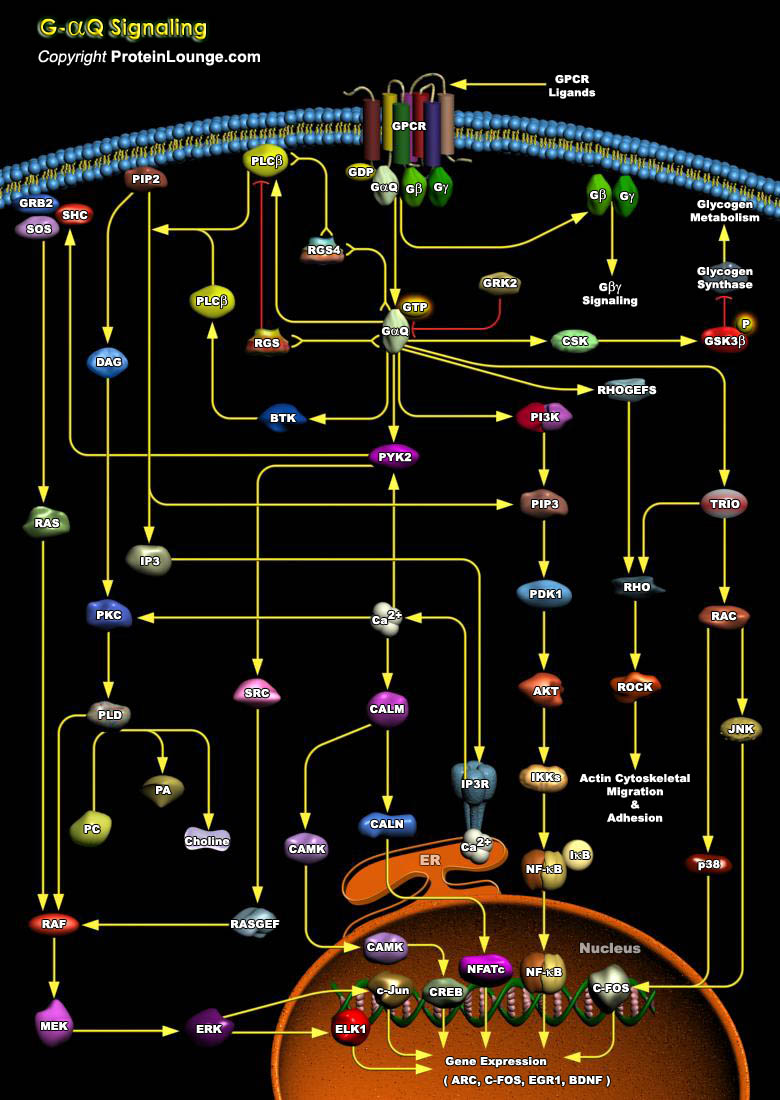
The heterotrimeric G-Proteins (Guanine nucleotide-binding Proteins) are signal transducers that communicate signals from many hormones, neurotransmitters, chemokines, and autocrine and paracrine factors. The extracellular signals are received by members of a large superfamily of receptors with seven membrane-spanning regions, known as GPCR (G-Protein Coupled Receptor), that activate the G-proteins, which route the signals to several distinct intracellular signaling pathways. These pathways interact with one another to form a network that regulates metabolic enzymes, ion channels, transporters, and other components of the cellular machinery controlling a broad range of cellular processes, including transcription, motility, contractility, and secretion. These cellular[..]
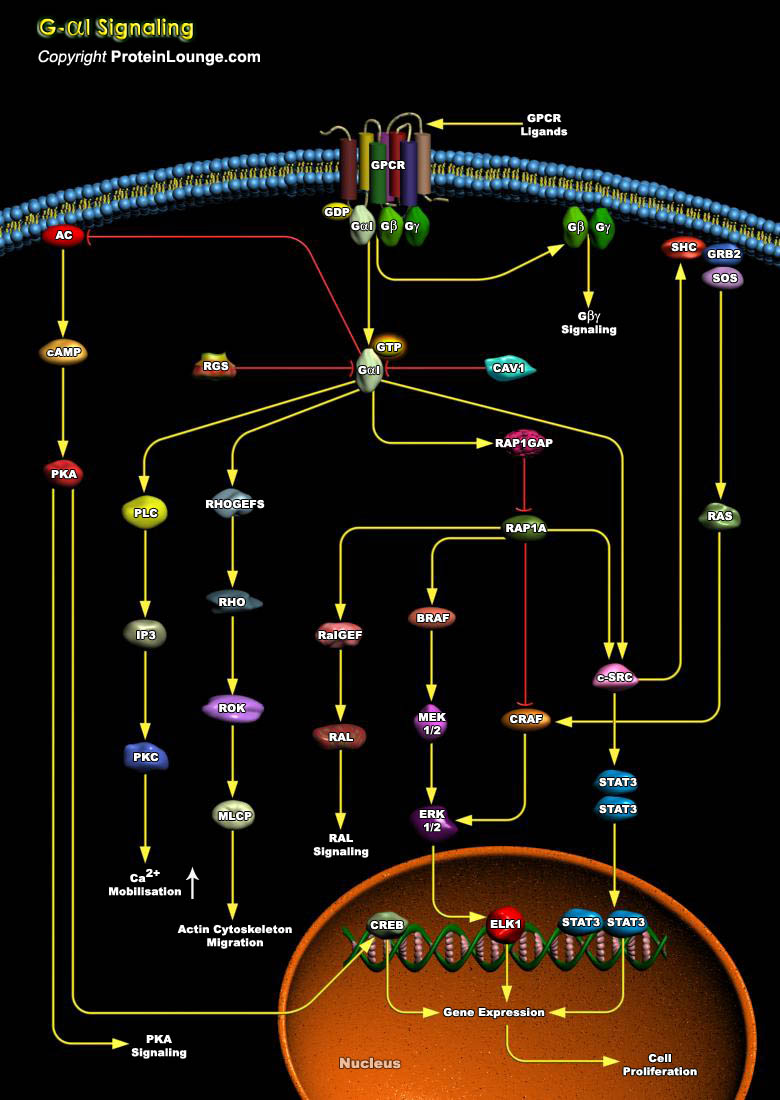
G-proteins (Guanine Nucleotide-Binding Proteins) are heterotrimeric proteins that mediate signal transduction between many membrane-bound receptors and intracellular effectors. Traditionally, activation of heterotrimeric G-proteins is accomplished exclusively by the action of GPCRs (G-Protein Coupled Receptors), Seven transmembrane-spanning proteins that typically reside in the Plasma membrane. G-proteins consist of Alpha, Beta and Gamma subunits, and each is involved in signaling to distinct effectors. There are 20 different mammalian G-Alpha subunits (depending on alternative splicing) and 6 Beta and 14 Gamma subtypes. The G-Beta-subunit of heterotrimeric G-proteins has a Beta-propeller structure containing seven WD-40 repeats. The Gamma-subunit interacts with the[..]
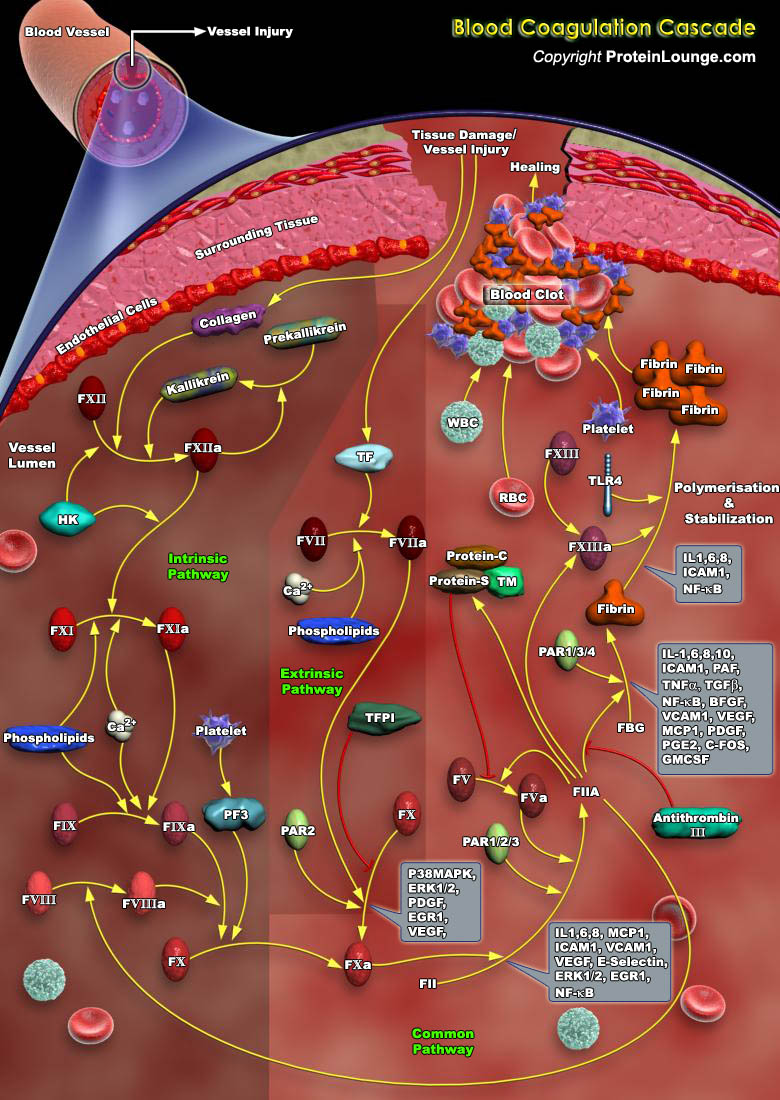
Coagulation is a dynamic process which involves the regulated sequence of proteolytic activation of a series of zymogens to achieve appropriate and timely haemostasis in an injured vessel, in an environment that overwhelmingly favours an anticoagulant state [Ref.1 & 2]. There are two main mechanisms for triggering the blood clotting, termed as the contact pathway/intrinsic pathway and the tissue factor pathway/extrinsic pathway [Ref.3].The contact pathway of coagulation is initiated by activation of factor XII (fXII) in a process that also involves high-molecular-weight kininogen (HK) and plasma prekallikrein (PK). Contact of blood with an artificial surface leads to a change in the conformation of fXII, resulting in the generation of small amounts of active factor XII[..]
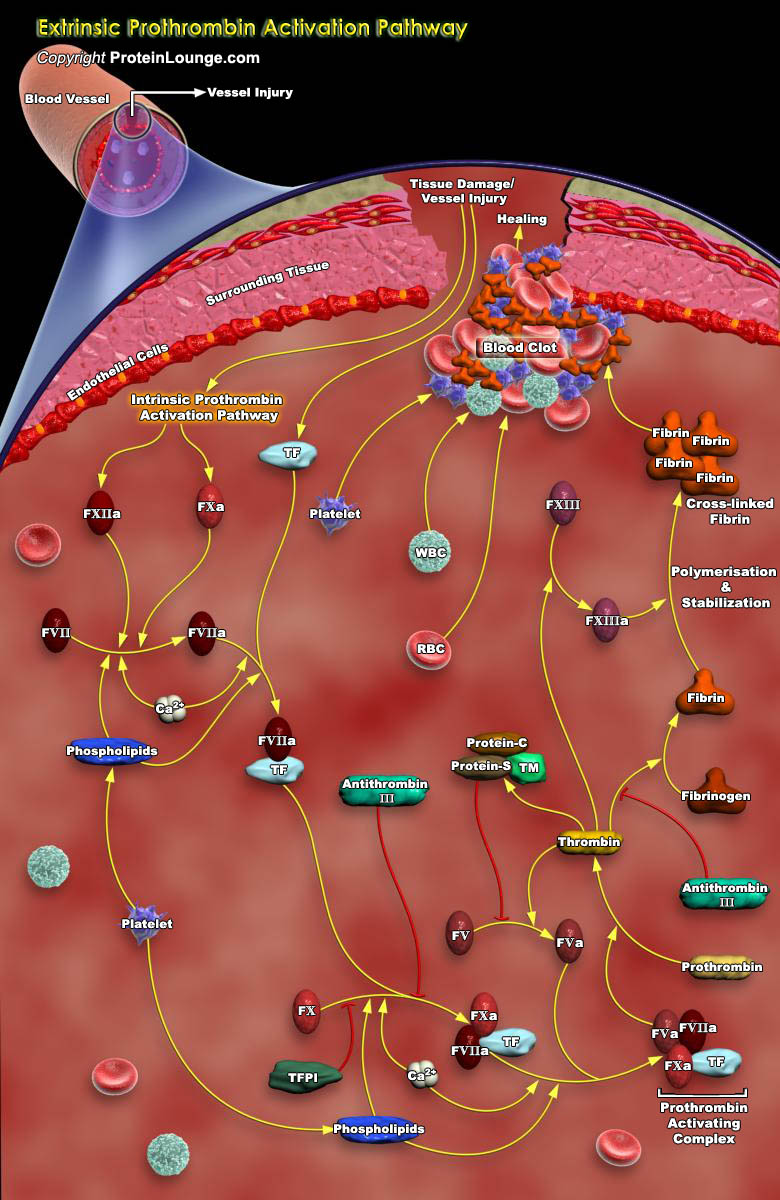
Thrombin/TFIIa (Activated Factor-II) is a coagulation protein that has many effects in the Coagulation cascade, the homeostatic process of greatest interest. It is a multifunctional serine proteinase best known for its ability to cleave Fibrinogen to Fibrin. Fibrin forms an essential component of the Blood Clot. When a blood vessel is injured, bleeding is stopped by clotting factors which form a Thrombus/Clot of Fibrin threads that trap platelet aggregates. A clot is a jelly-like mass of thickened blood composed of Fibrin and platelet aggregates. The first step in clotting is adhesion of platelets, which are fragments of blood cells that circulate in the blood, to the cut edges of a damaged blood vessel. In this way, a platelet plug is formed and external bleeding[..]
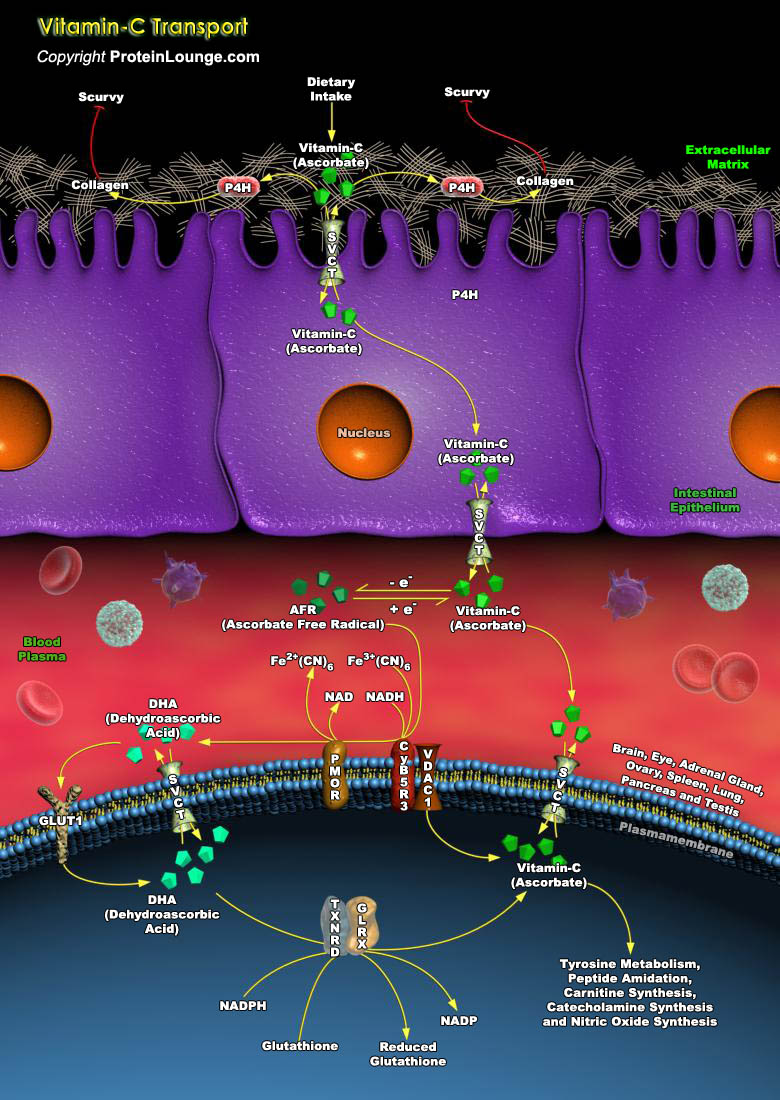
Vitamin-C (Ascorbate or Ascorbic Acid) is an essential water-soluble Vitamin, well known for its antiscorbutic and antioxidant functions in humans. Vitamin-C was first identified by virtue of the essential role it plays in Collagen modification, preventing the nutritional deficiency Scurvy. Vitamin-C acts as a cofactor for the P4H (Prolyl Hydroxylase) enzymes, which post-translationally modify Collagen and thereby increase the strength and elasticity of tissues. Vitamin-C reduces the metal ion prosthetic groups of many enzymes, thereby maintaining the activity of enzymes. The fact that prevention of Scurvy through modification of Collagen is the most obvious role for Vitamin-C it is not necessarily the only role of Vitamin-C (Ref.1). Collagen modification depends on[..]
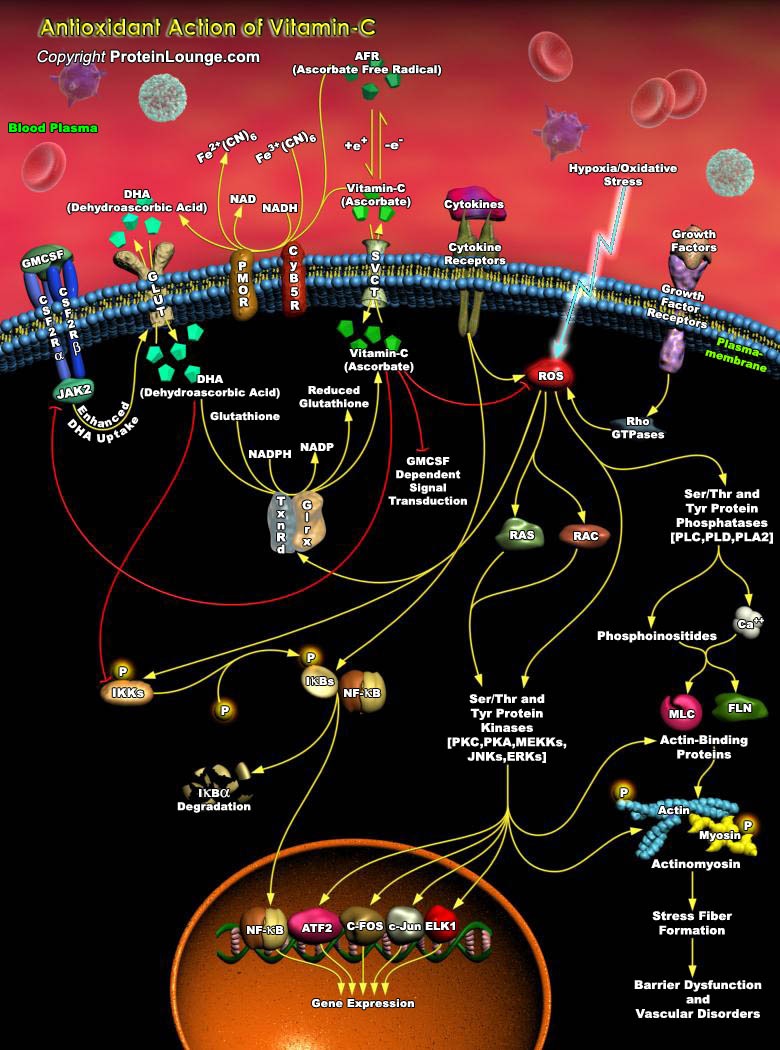
Oxidative stress/Hypoxia is induced by a wide range of environmental factors including UV stress, pathogen invasion (hypersensitive reaction), oxygen shortage, etc. Generation of ROS (Reactive Oxygen Species) is characteristic feature of such stress conditions. Of the ROS, both Hydrogen Peroxide and Superoxide are produced in a number of cellular reactions and by various enzymes such as Lipoxygenases, Peroxidases, NADPH Oxidase, Xanthine Oxidase, etc to name a few. The main cellular components susceptible to damage by free radicals are lipids (peroxidation of unsaturated fatty acids in membranes), proteins (denaturation), carbohydrates and nucleic acids. Consequences of oxidative stress depend on tissue and/or species (i.e. their tolerance to Anoxia), on membrane[..]
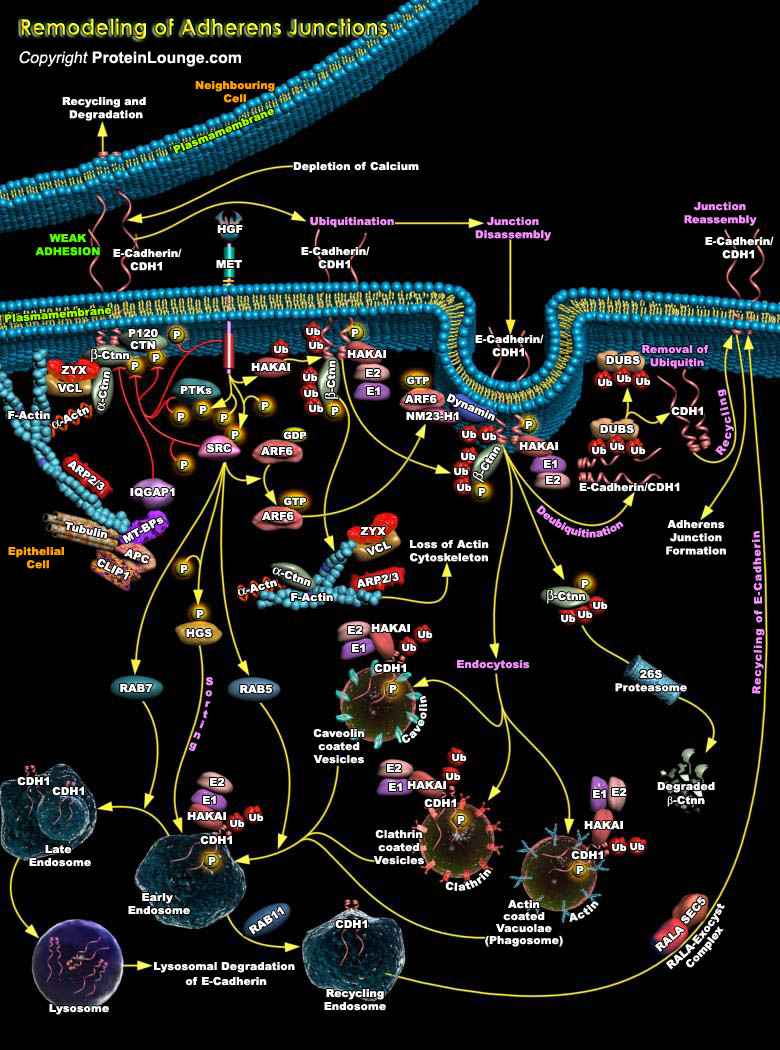
The intercellular Adherens Junctions (AJs) are specialized sub-apical structures that function as principle mediators of cell-cell adhesion. Their disassembly correlates with a loss of cell-cell contact and an acquisition of migratory potential. The Adherens Junctions have a crucial role both as sensors of extracellular stimuli and in regulating the dynamics of epithelial cell sheets or with neighboring cells. Cadherins, the Type-I transmembrane proteins of the Adherens Junctions, are principally responsible for homotypic cell-cell adhesion. E-Cadherin, which is present primarily in epithelia, is the best-characterized Cadherin and represents the prototype of classical Cadherins. The extracellular domain of E-Cadherin binds to Ca2+ (Calcium) and forms complexes with[..]
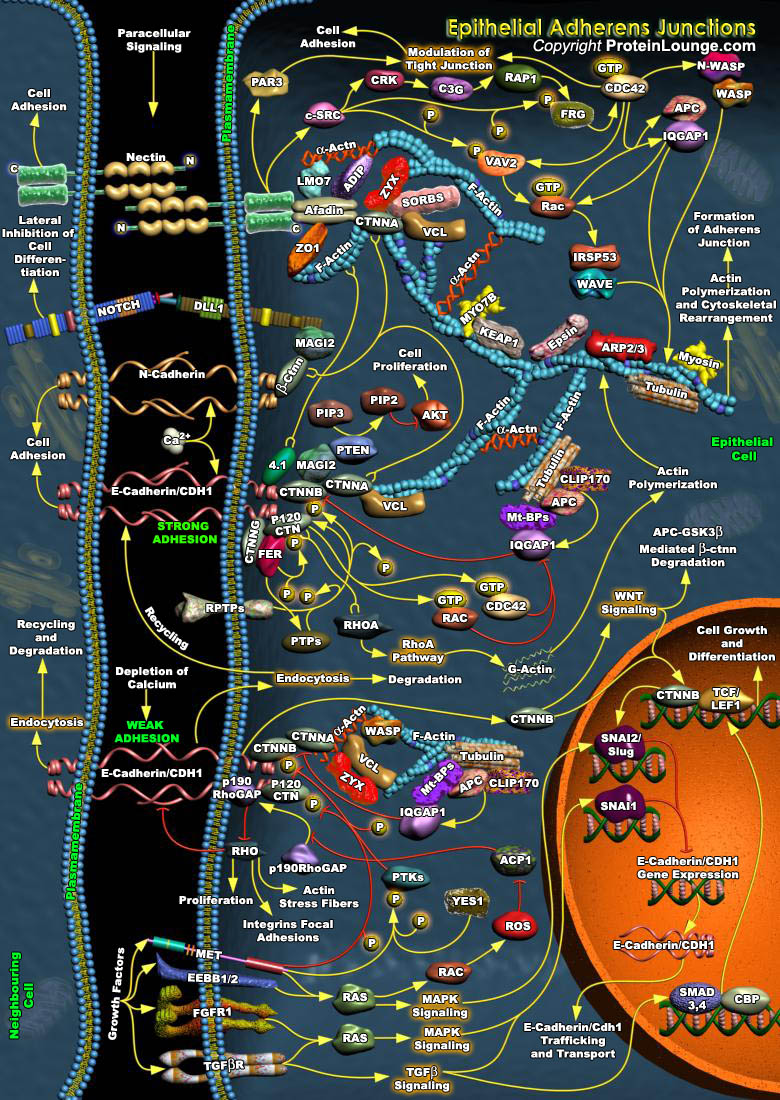
Adhesion between neighboring epithelial cells is a crucial and tightly controlled process. The integrity of cell-cell contacts is essential for the regulation of electrolyte absorption and for the prevention of tumor metastasis. In polarized epithelia, specialized structures such as Adherens Junctions (AJs) and Tight Junctions (TJs) are responsible for the establishment of contacts between neighboring cells. The establishment and stability of Adherens Junction is tightly regulated-in particular, by Growth Factors, cytokines and hormones. Such regulation, although poorly understood, is quite essential for the modulation of paracellular permeability in various epithelia, for the epithelium mesenchyme transition, and for development, morphogenesis and wound healing[..]
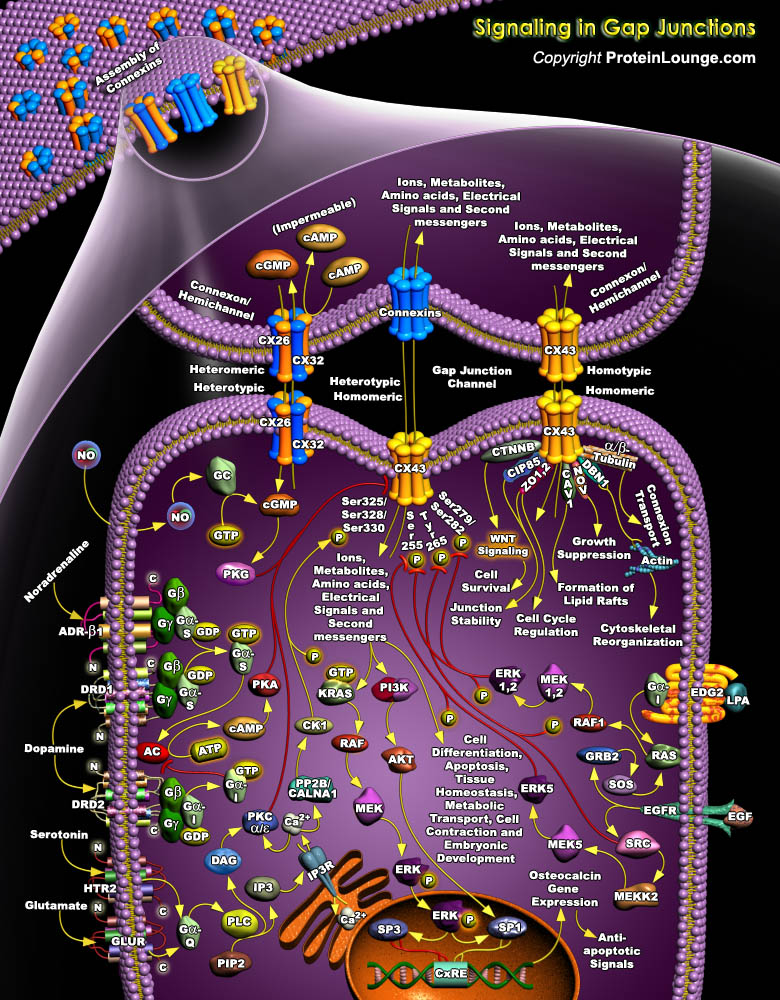
Gap Junction (GJ) channels span two plasma membranes and are formed by the alignment of two hemichannels, each consisting of an oligomer of structural subunit proteins, called Cxs (Connexins). These junctional proteins constitute a multigene family whose members are distinguished according to their predicted molecular weight in kilodaltons. A Connexin structure consists of two extracellular loops (EL), four membrane-spanning domains (TM), one cytoplasmic loop (CL), one N-terminal tail (NT), and one C-terminal tail (CT) (Ref.1 & 2). During intercellular channel formation, six Connexins oligomerize into a Connexon or hemichannel that docks in homotypic, heterotypic and combined heterotypic/heteromeric arrangements. In total, as many as 14 different Connexon[..]
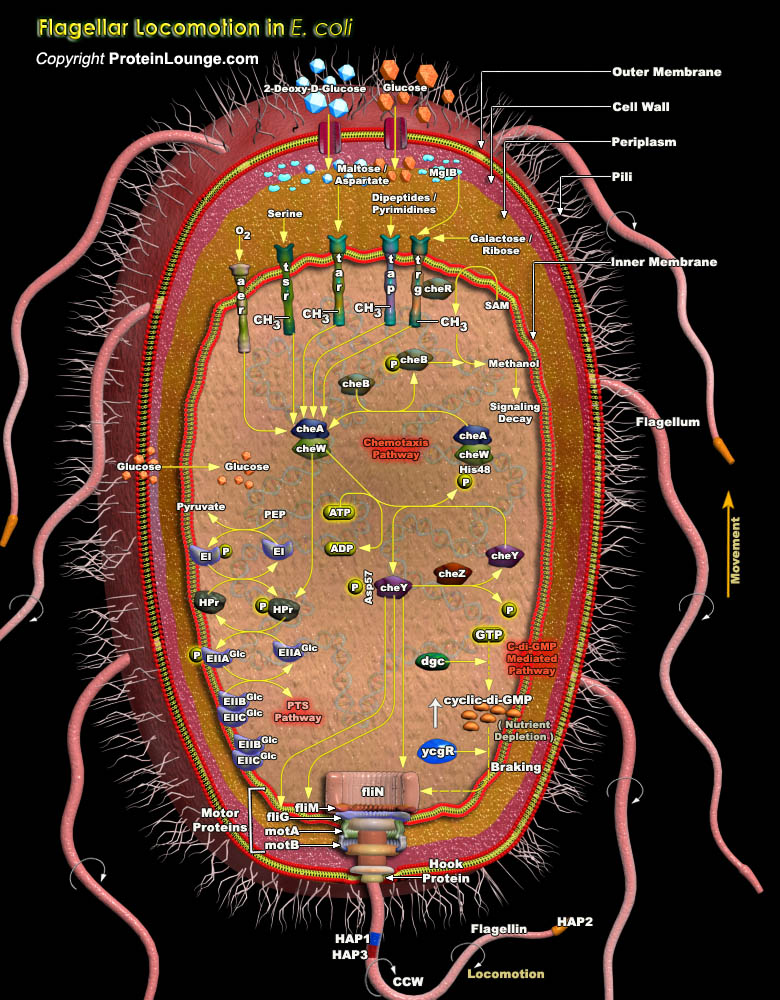
In an ever-changing environment, it is essential that organisms are able to sense these changes and to respond appropriately. Possible responses include alterations in gene expression and/or active movement towards or away from an environment. Most sensory pathways in eukaryotic organisms rely on serine, threonine or tyrosine protein kinases, whereas the most common sensory pathways in prokaryotes use a HAP (Histidine-Aspartate Phosphorelay) system. HAP systems have at least two components-a dimeric HPK (Histidine Protein Kinase) and a RR (Response Regulator). HAP systems are also found in many lower eukaryotes. Bacteria can sense a vast range of environmental signals, from the concentrations of nutrients and toxins to oxygen levels, pH, osmolarity and the intensity[..]

