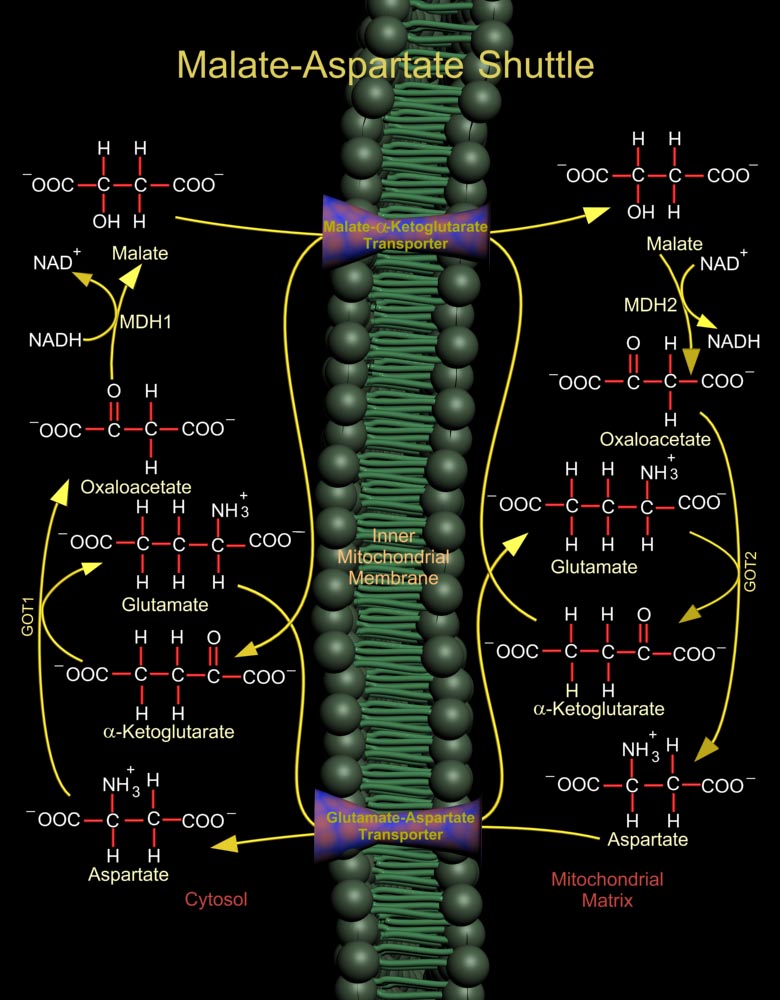
The Malate (L-Malic Acid)-Asp (Aspartate or L-Aspartate or Aspartic Acid) Shuttle of mammalian systems is more complex but more energy efficient. Mitochondrial NAD+ (Nicotinamide Adenine Dinucleotide) is reduced by cytosolic NADH (Nicotinamide Adenine Dinucleotide, Reduced) through the intermediate reduction and subsequent regeneration of OAA (Oxaloacetate) (Ref.1). In the cytosol, the shuttle converts OAA to Malate using the enzyme MDH1 (Malate Dehydrogenase-Cytoplasmic), and at the same time re-oxidizes NADH to NAD+, making it available for Glucose oxidation. Malate is then shuttled into the mitochondria by a Malate-Alpha-Ketoglutarate Transporter. In the mitochondria, the reverse reaction takes place and Malate is again converted to OAA by the enzyme MDH2 (Malate[..]
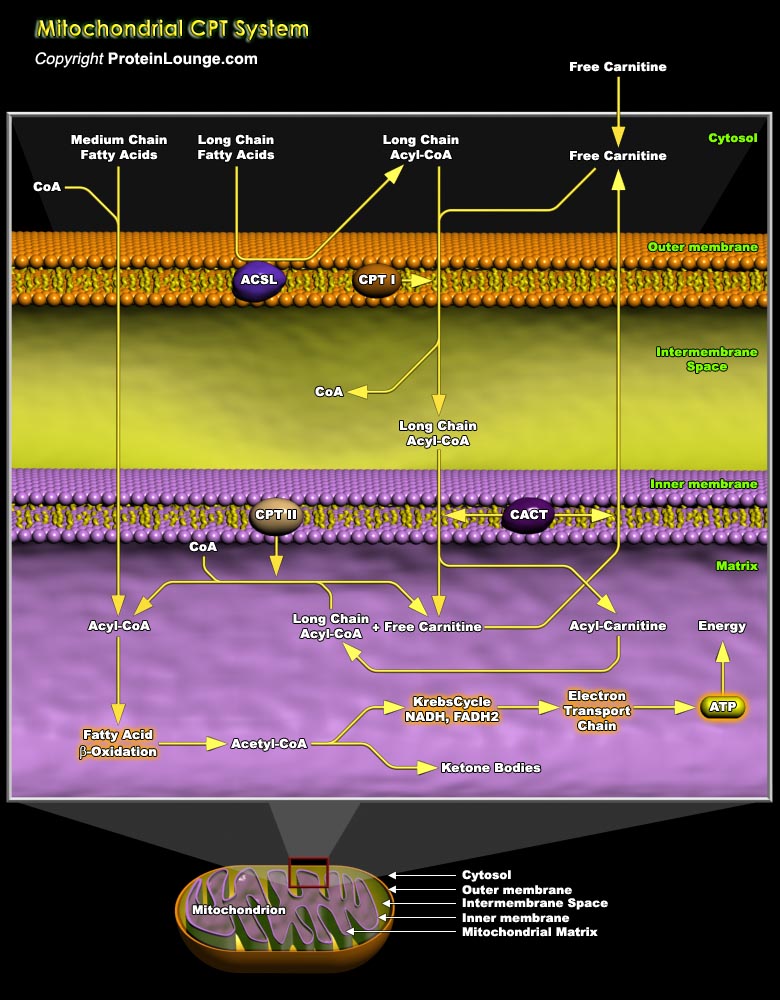
Mitochondrial fatty acid oxidation (FAO) and its key rate-limiting enzyme, the carnitine palmitoyltransferase (CPT) system, are important for energy homeostasis in situations like fasting or during exercise. They also regulate host immune responses and the deficiency or over-activation of CPT may cause energy metabolism disorder that may lead to many diseases starting from inflammatory disorders to cancer. Fatty acid catabolism occurs mostly in mitochondria through the beta-oxidation pathway (Ref.1 and 2). Long-chain fatty acids (LCFA), major component of fatty acids, that are important source of energy for heart, liver and muscles, cannot enter the mitochondria by simple diffusion unlike short or medium chain fatty acids. LCFA are activated and converted to[..]
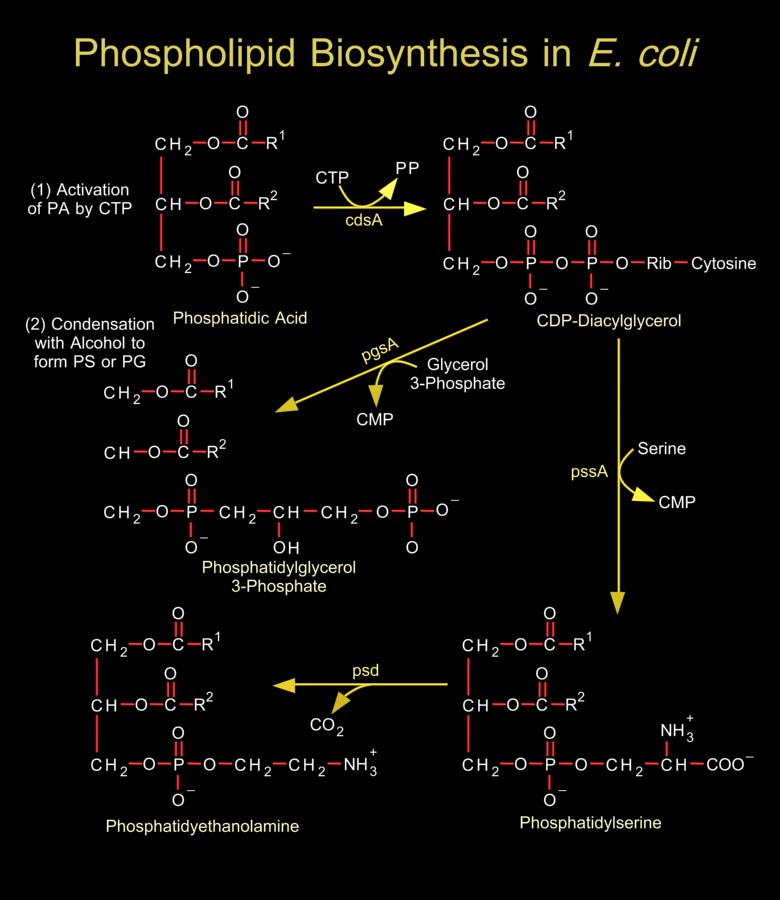
Biological membranes are composed of hundreds of distinct proteins and phospholipids. Phospholipids are diacylglycerol derivatives with a hydrophilic, zwitter ionic, often charged headgroup at position C3 of the glycerol backbone. The properties of phospholipids give lipid bilayer membranes their self-organizing structure. Phospholipids are usually composed of two fatty acid chains esterified to two of the carbons of glycerol phosphate, the phosphate being esterified to a hydroxyl group of another hydrophilic compound, such as choline, ethanolamine or serine.In general, E. coli and most related gram-negative bacteria do not contain phosphatidylcholine, phosphatidylinositol, sphingolipids, glycolipids, plasmalogens, or sterols, which are characteristic of[..]
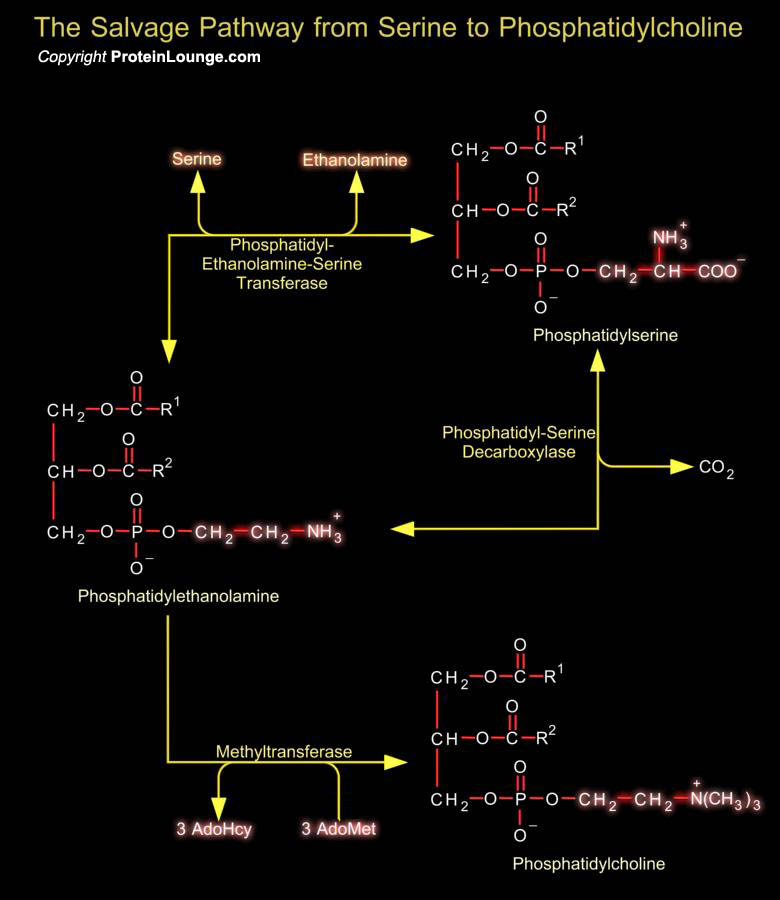
Phosphatidylserine (or 1, 2-Diacyl-sn-Glycero-3-Phospho-L-Serine) is the only amino acid-containing glycerophospholipid in animal cells. Although it is distributed widely among animals, plants and microorganisms, it is usually less than 10% of the total phospholipids, the greatest concentration being in myelin from brain tissue. It is an acidic (anionic) phospholipid with three ionizable groups, i.e. the phosphate moiety, the amino group and the carboxyl function. As with other acidic lipids, it exists in nature in salt form.In bacteria and other prokaryotic organisms, Phosphatidylserine is synthesized by a mechanism comparable to that of other phospholipids, i.e. by reaction of L-Serine with CDP-DAG (CDP-Diacylglycerol).In contrast in animal tissues, there are two[..]
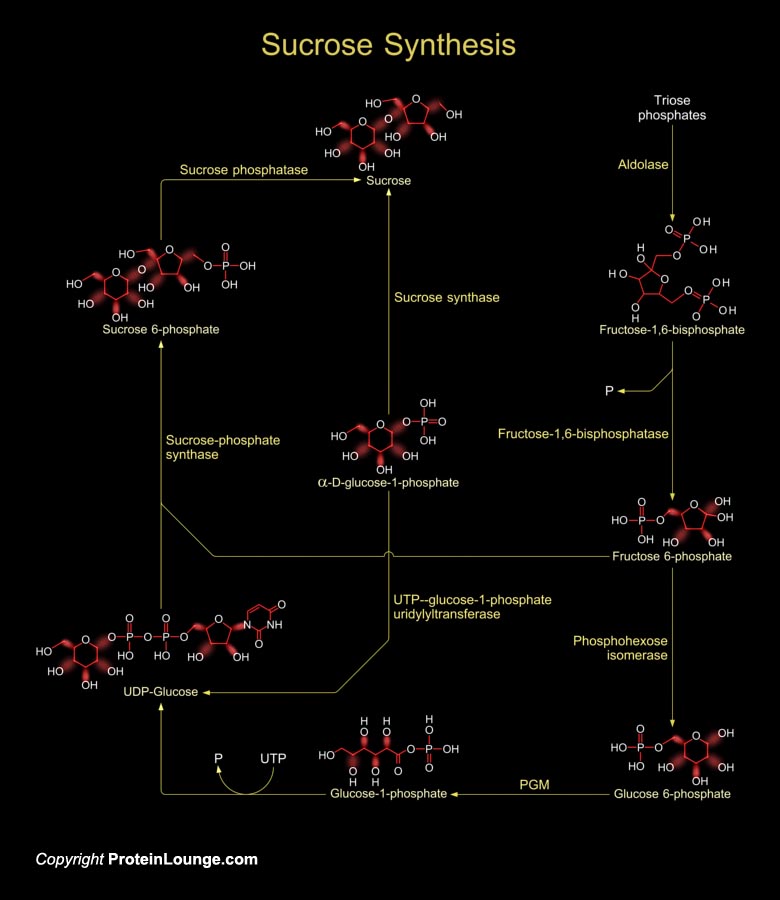
Sucrose is produced by plants, algae and cyanobacteria as an end product of photosynthesis. It is the primary sugar transported from the source tissues to sink tissues by the phloem in most plants. In other non-photosynthetic tissues, sucrose serve as raw material for many metabolic pathways (Ref.1).Sucrose synthesis starts in the cytosol where two triose-phosphates produce one Fructose-1,6-bisphosphate by the enzyme aldolase. Fructose-1,6-bisphosphate is converted to fructose-6-phosphate by the enzyme Fructose-1,6-bisphosphatase. Then the enzyme phosphohexose isomerase converts fructose-6 phosphate to glucose-6-phosphate. Glucose-6-phosphate then forms glucose-1-phosphate in presence of the enzyme phosphoglucomutase (PGM). The glucose-1-phosphate is converted to[..]
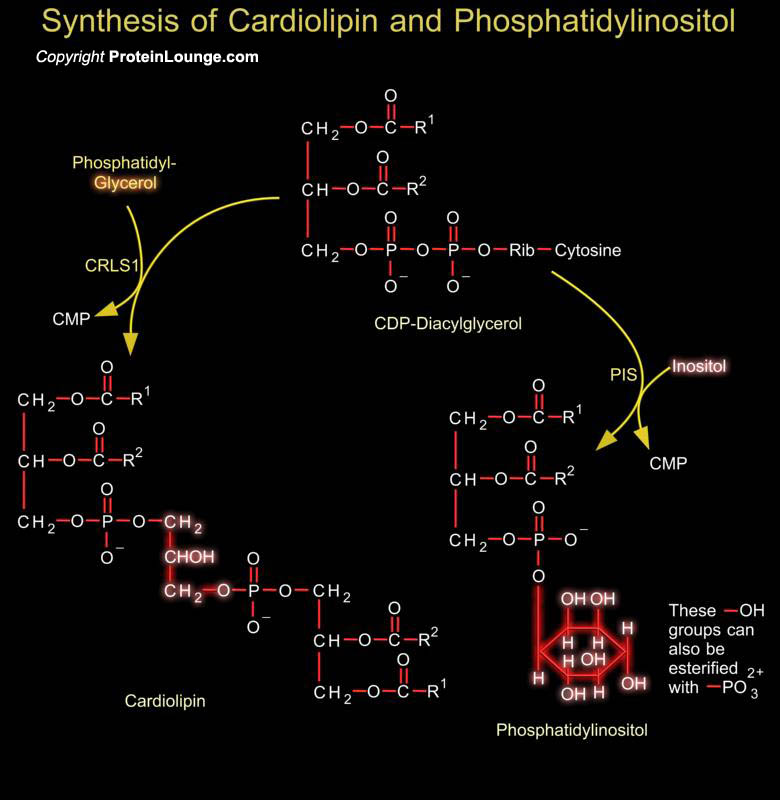
Phosphoglycerides such as phosphatidylethanolamine and phosphatidylserine are the major component of lipid bilayer membranes in which their detergent-like properties help to provide membranes the quality of self-assembly. Phosphatidylglycerol is a precursor in the synthesis of both cardiolipin and PI (Phosphatidylinositol). Cardiolipin is a unique dimeric phospholipid found in the heart and most mammalian tissues. This phospholipid is the only phospholipid localized primarily in the mitochondrial membrane of mammalian cells. In cardiolipin, two phosphatidylglycerol units are joined together. Phosphatidic acid is activated to CDP-DAG (CDP-Diacylglycerol), which is the precursor to PI and cardiolipin. PI is the precursor of PI Phosphates, which have one of more[..]
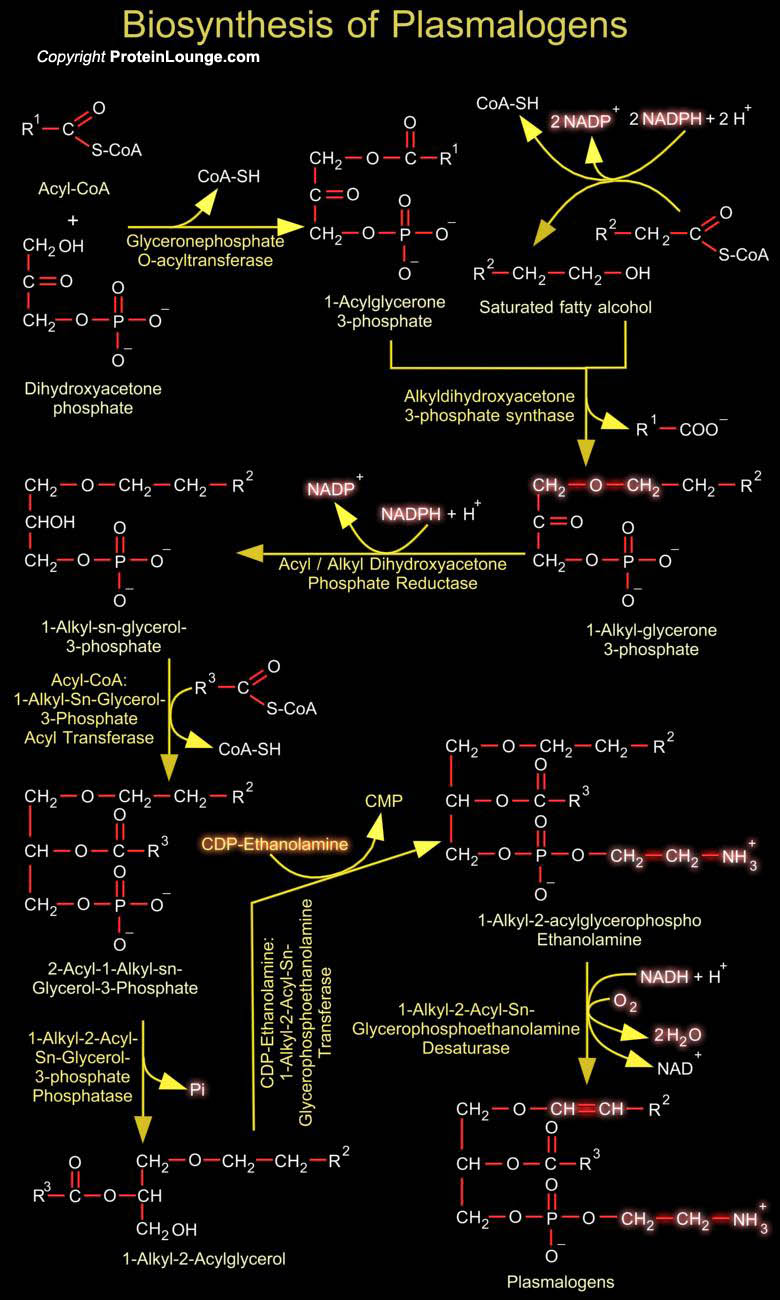
Plasmalogens are glycerophospholipids of neural membranes containing vinyl ether bonds. Their synthetic pathway is located in peroxisomes and endoplasmic reticulum. About 20% of mammalian Glycerophospholipids are Plasmalogens. The exact percentage varies both from species to species and from tissue to tissue within a given organisms. While Plamalogens comprise only 0.8% of phospholipids in human liver, they account for 23% of those in human nervous tissue (Ref.1). One of the starting materials for plasmalogen biosynthesis is DHAP (Dihydroxyacetone Phosphate) from glycolysis, which is used to form the glycerol backbone of the plasmalogen. The initial steps of plasmalogen biosynthesis are catalyzed by DHAP-AT (Dihydroxyacetone phosphate Acyltransferase) and[..]
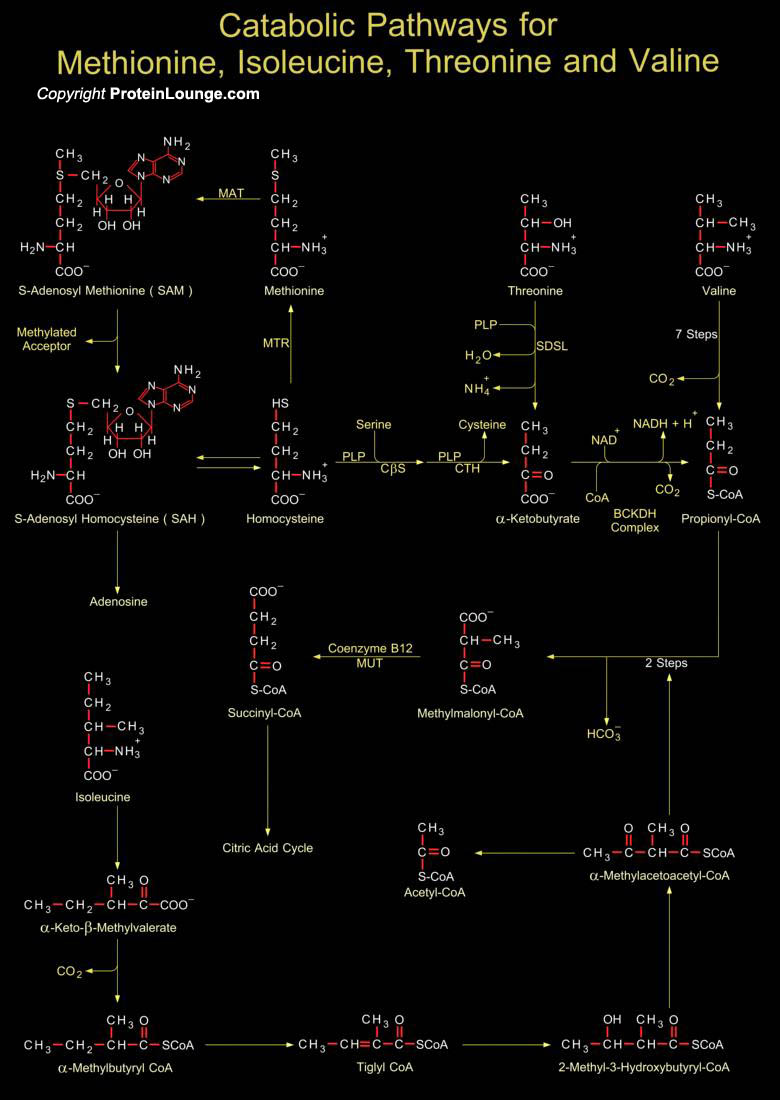
The carbon skeletons of Methionine, Isoleucine, Threonine and Valine are degraded by pathways that yield Succinyl-CoA (Succinyl-Coenzyme A), an intermediate of the Citric Acid Cycle. Methionine donates its Methyl group to one of several possible acceptors through S-adenosylmethionine and three of its four remaining carbon atoms are converted to the Propionate of Propionyl-CoA, a precursor of Succinyl-CoA. Isoleucine undergoes transamination, followed by oxidative decarboxylation of the resulting Alpha-Keto Acid. The remaining five-carbon skeleton is further oxidized to Acetyl-CoA and Propionyl-CoA (Ref. 3). Valine undergoes transamination and decarboxylation and then a series of oxidation reactions convert the remaining four carbons to Propionyl-CoA (Ref.1). In[..]
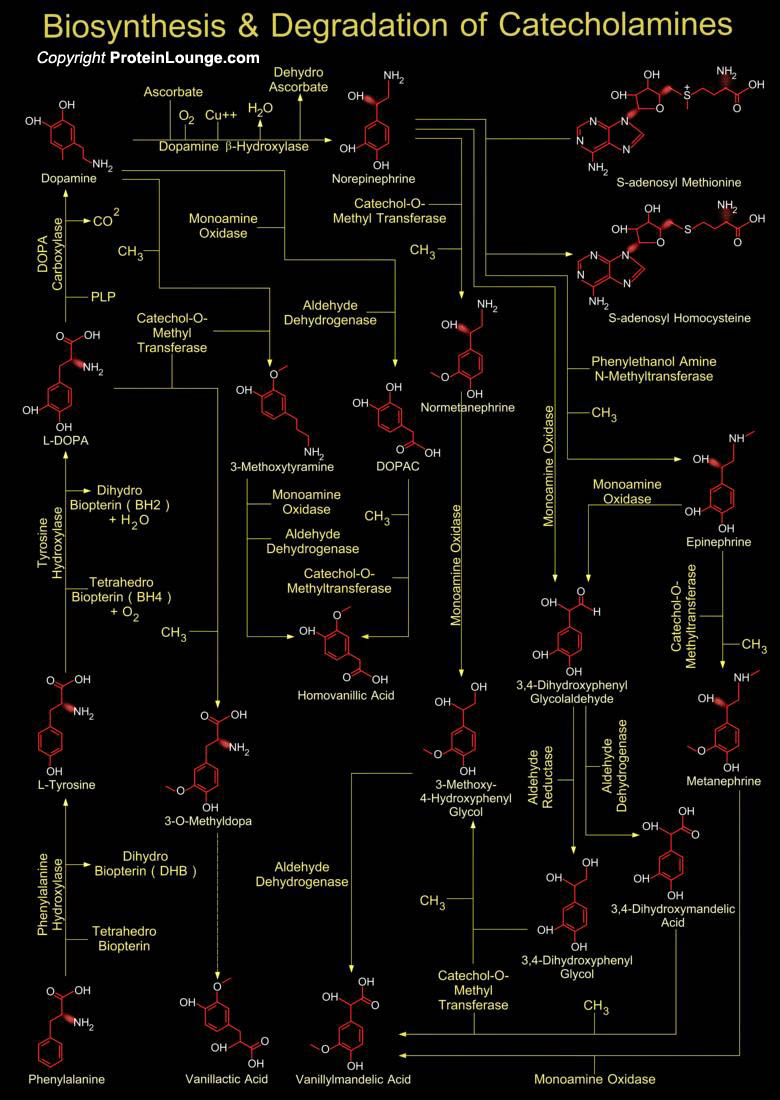
Catecholamine is the common term for the important hormones Norepinephrine (Noradrenaline), Epinephrine (Adrenaline) and Dopamine. Chromaffin cells of the adrenal medulla are the key site of catecholamine synthesis and collections of these cells are also found in heart, liver, kidney, gonads, adrenergic neurons of the postganglionic sympathetic system, and CNS (Central Nervous System). The major product of the adrenal medulla is epinephrine, which constitutes 80% of the catecholamines released from the medulla (Ref.1&2). Tyrosine initiates catecholamine biosynthesis. It is produced in the liver from phenylalanine through the action of phenylalanine hydroxylase. The tyrosine is then transported to catecholamine-secreting neurons where a series of reactions[..]
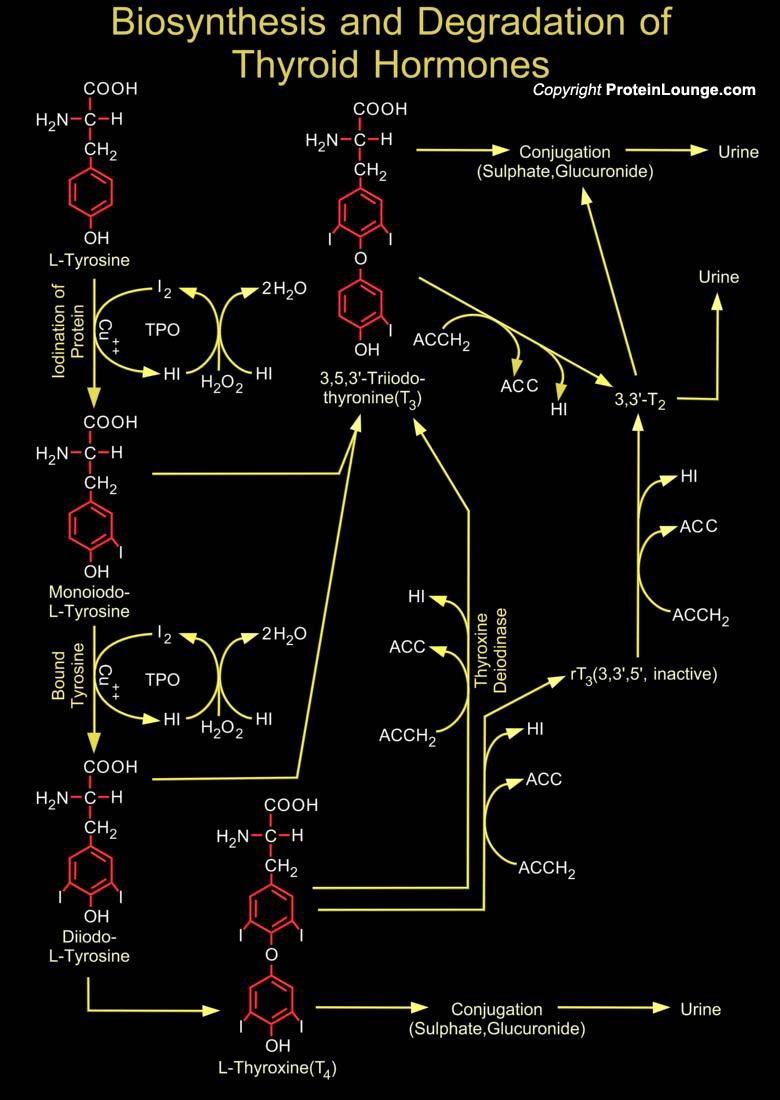
Iodide, which is ingested in food and water, is actively concentrated by the thyroid gland, converted to organic iodine by TPO (Thyroid Peroxidase also known as Iodide Peroxidase), and incorporated into tyrosine in intrafollicular thyroglobulin within the colloid at the basal cell surface of the thyroid follicular cell. The tyrosines are iodinated at one (Monoiodotyrosine) or two (Diiodotyrosine) sites and then coupled to form two active hormones, Thyroxine (T4, a Tetra-Iodinated Tyrosine derivative) and Triiodothyronine (T3). Unlike the other endocrine hormones, these are not peptides. They are derived from the amino acid tyrosine. T4 is the major active hormone. In tissues outside the thyroid, particularly in the liver and kidney, T4 is converted to T3, an active[..]
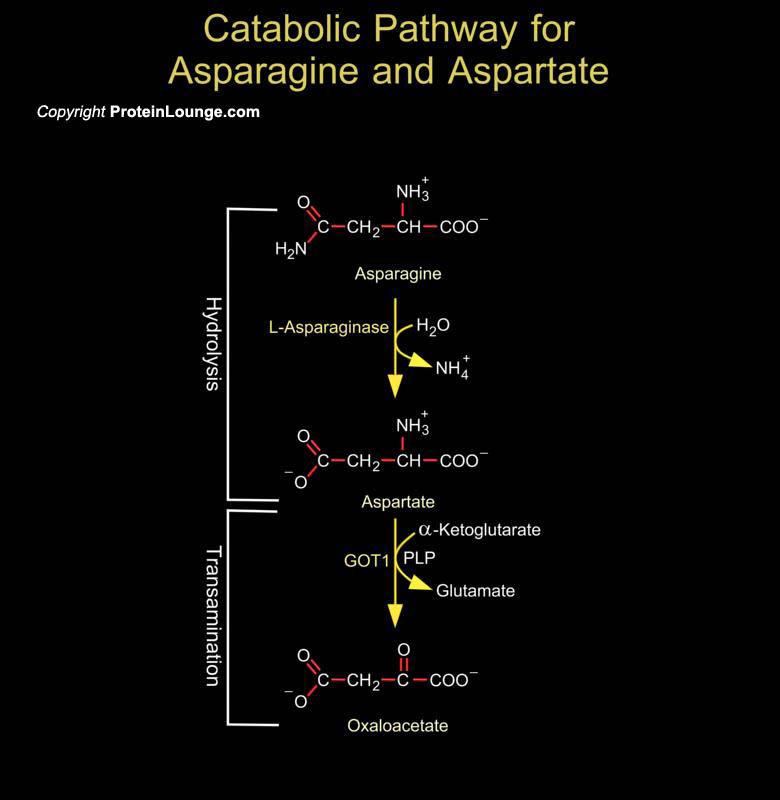
Asparagine, a non essential amino acid, synthesized form oxaloacetic acid. It is an amide of a amino acid Aspartate. The enzymes ASPG (asparaginase) and ASRGL1 (asparaginase and isoaspartyl peptidase 1) catalyze the hydrolysis of Asparagine to Aspartate and Ammonia. The enzyme GOT1 (glutamic-oxaloacetic transaminase 1) catalyzes the transamination of Aspartate to oxaloacetic acid which can subsequently enter either the TCA cycle or gluconeogenesis. ASPG play a prominent role in chemotherapy, as cancer cells are dependent on the availability of extracellular Asparagine(Ref.1 &2). Aspartate is converted to Asparagine in an ATP-dependent amidotransferase reaction a[..]
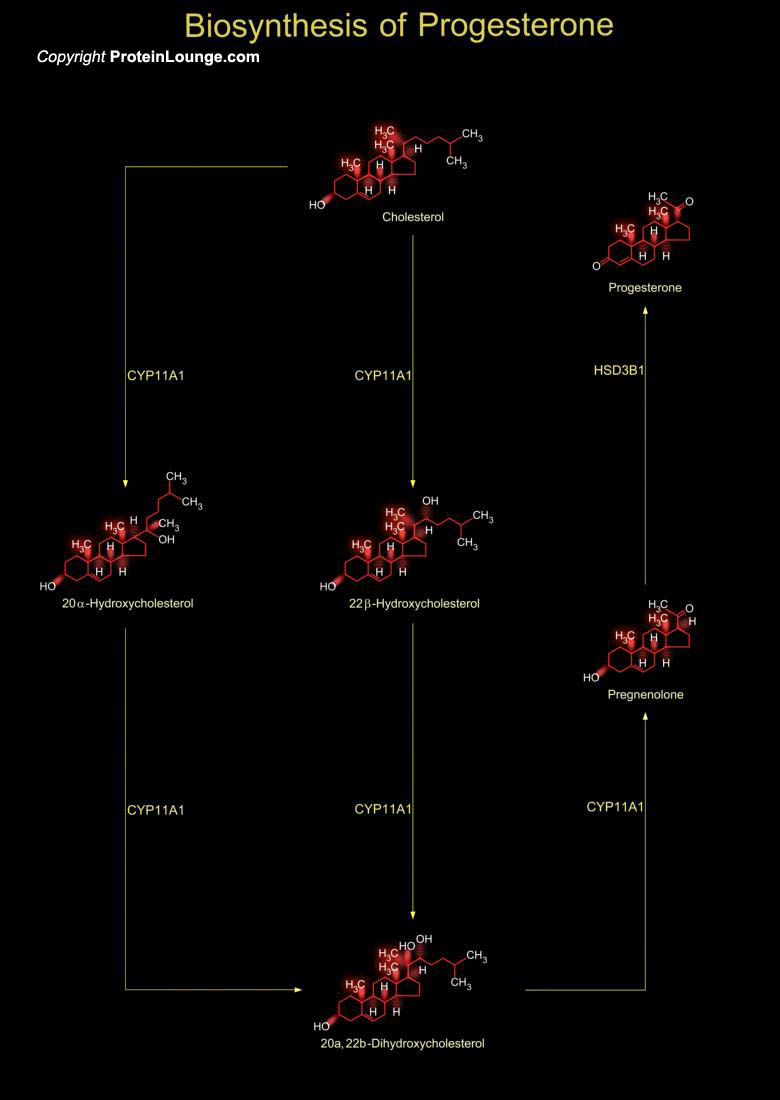
Cholesterol is the common precursor of all steroid hormones and is biosynthesized in many tissues. Steroid hormones are characterized by a common basic structure of cyclopentane-perhydro-phenantrene, a polycyclic complex of 17 carbon atoms forming a four-ring system. Altering a side chain or subsistent group of a cholesterol molecule produces various steroids molecules. Pregnenolone and progesterone are molecules derived from cholesterol (Ref.1&2).Cholesterol is converted to pregnenolone inside the mitochondria by the enzyme CYP11A1 (cytochrome P450 family 11 subfamily A member 1). This conversion is the first step in the synthesis of steroids and is termed as the cholesterol side-chain cleavage reaction. Pregnenolone then passes through the mitochondrial[..]

