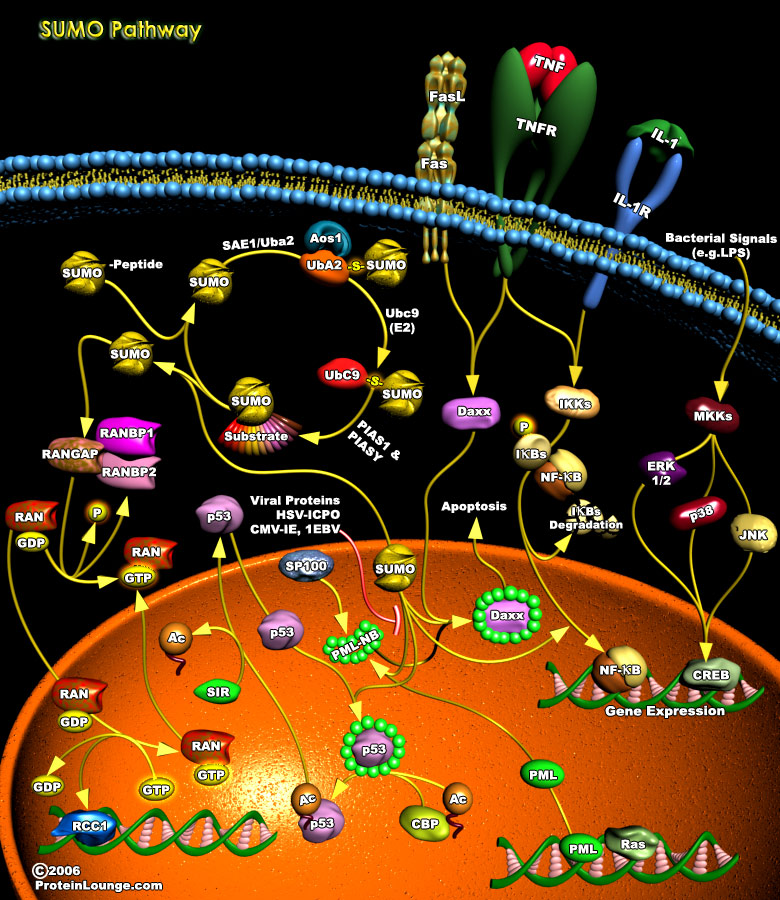
Covalent modifications of proteins, such as phosphorylation, acetylation and ubiquitylation, play an important role in most cellular processes because they can cause rapid changes in the activities of pre-existing proteins. This type of mechanism for regulating protein function is especially crucial in signal transduction pathways and in cell cycle. Posttranslational modifications (PTMs) by members of the ubiquitin family are covalent events that promote radical changes in the properties of modified proteins. Among all ubiquitin-like molecules, a particular attention has been given to the modification by SUMO (Small Ubiquitin Modifier) also known as Sentrin. SUMOylation plays critical roles in a variety of cellular processes, including transcription, cellular[..]
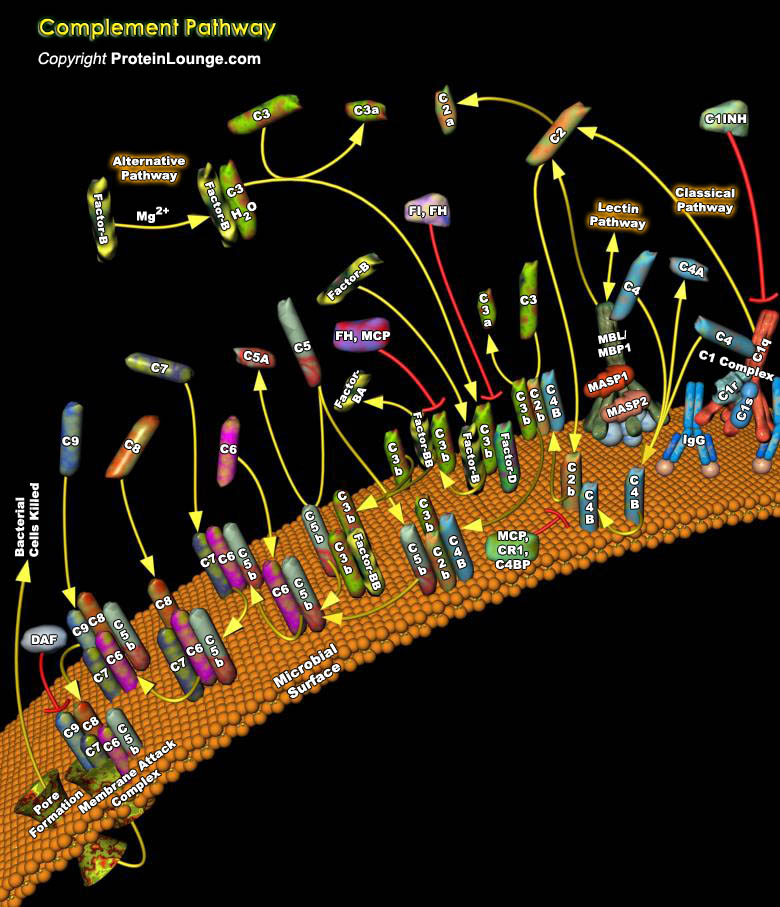
The C (Complement) system consists of about twenty plasma proteins that function either as enzymes or as binding proteins. In addition to these plasma proteins, the complement system includes multiple distinct cell-surface receptors that exhibit specificity for the physiological fragments of complement proteins and that occur on inflammatory cells and cells of the immune system. Activation of the complement system initiates a series of enzymatic reactions in which the proteolytic cleavage and activation of successive complement proteins leads to the covalent bonding or fixing of complement fragments to the pathogen surface. A major consequence of this is the uptake and destruction of complement coated microbes by phagocytes that bear receptors for these complement[..]
 Pathway.jpg)
Nuclear factor-kappaB (NF-kappaB) comprises a family of transcription factors that regulate the expression of genes encoding proteins and microRNAs (miRNA, miR) precursors and may regulate a variety of biological processes such as inflammation, immunity, cell proliferation, differentiation, survival, cancer development, and progression (Ref.1, 2 and 3). NF-kappaB is ubiquitously expressed and responds to diverse stimuli, including infectious agents, cytokines, or growth factors. NF-kappaB /Rel family include five members, namely RELA (p65), RELB, c-REL, p50, and p52, all comprising a conserved REL homology domain (RHD) near the N-terminus, which are able to form homo- or heterodimers with the ability to transmit receptor signals to the nucleus. Although NF-kappaB is[..]
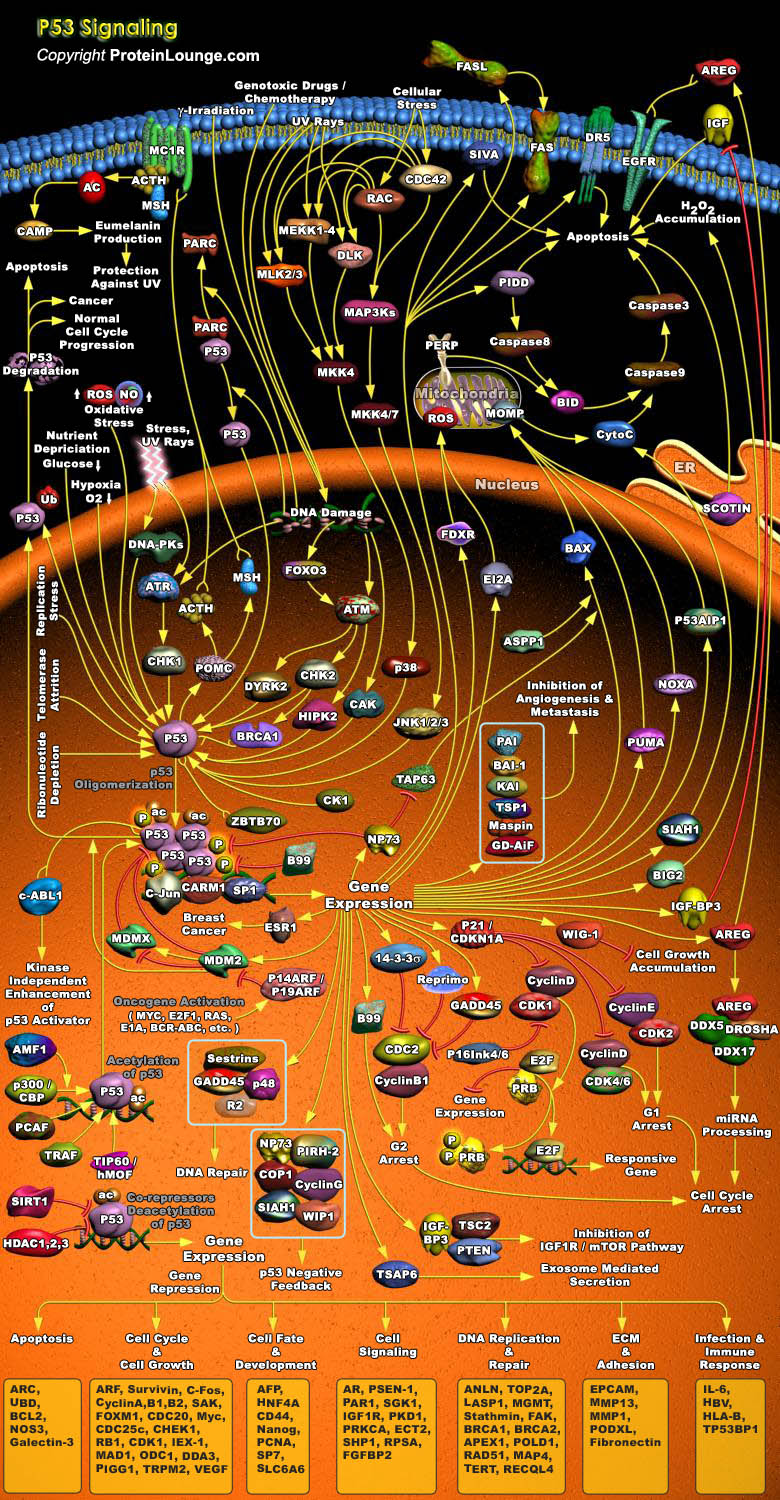
p53 is a tumour suppressor protein that regulates the expression of a wide variety of genes involved in Apoptosis, Growth arrest, Inhibition of cell cycle progression, Differentiation and accelerated DNA repair or Senescence in response to Genotoxic or Cellular Stress. As a transcription factor, p53 is composed of an N-terminal Activation Domain, a central specific DNA Binding Domain, and a C-terminal Tetramerization Domain, followed by a Regulatory Domain rich in basic Amino acids. Having a short half-life, p53 is normally maintained at low levels in unstressed mammalian cells by continuous ubiquitylation and subsequent degradation by the 26S Proteasome. Nonphosphorylated p53 is ubiquitylated by the MDM2 (Mouse Double Minute-2) ubiquitin ligase. MDM2 binding[..]
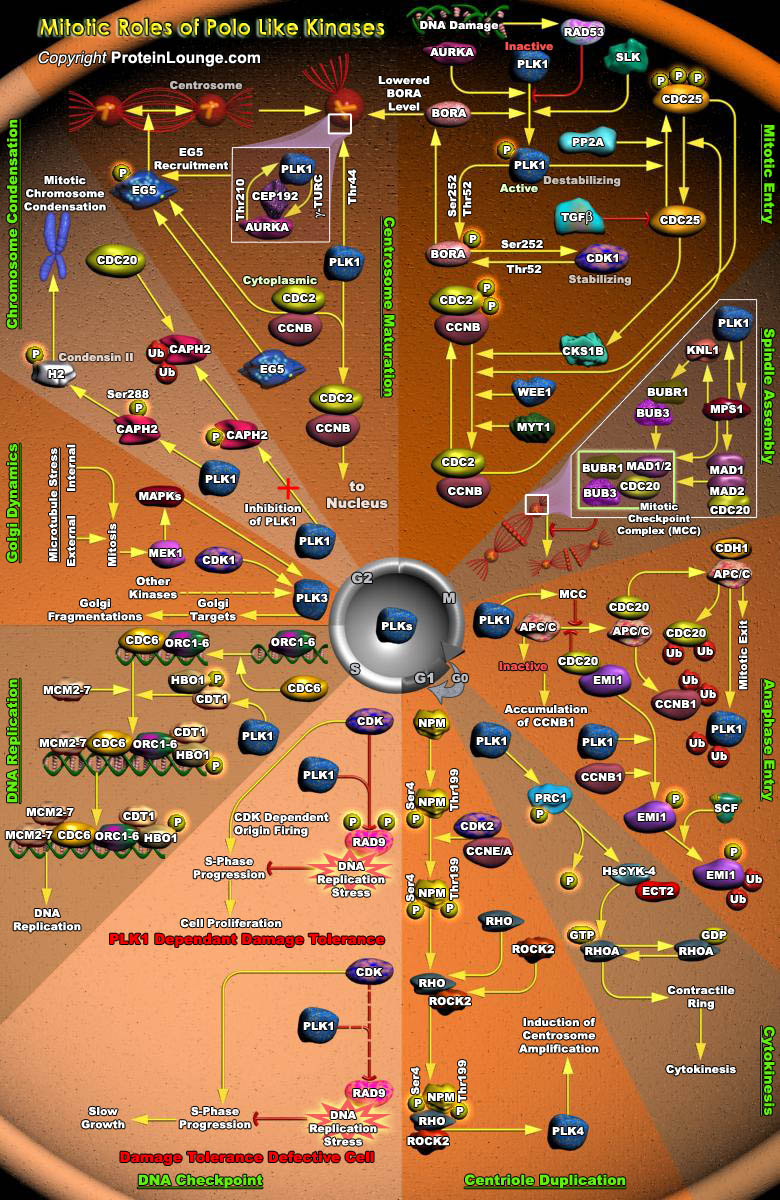
Cell division is characterized by orchestrated events of chromosome segregation, distribution of cellular organelles, and the eventual partitioning and separation of the two daughter cells. Mitosis is a highly regulated process that assures the proper allotment of genetic material between each pair of daughter cells. It proceeds through successive stages of well-defined and coordinated sub-processes. Entry into mitosis is regulated by the CDC2 (Cell Division Cycle-2)/Cyclin-B heterodimer. CDC2/Cyclin-B activity drives the events of early mitosis, such as nuclear breakdown, chromosome condensation and spindle formation by phosphorylating cellular substrates. While CDC2 (the catalytic subunit of the heterodimer) is required to drive the events of early mitosis,[..]
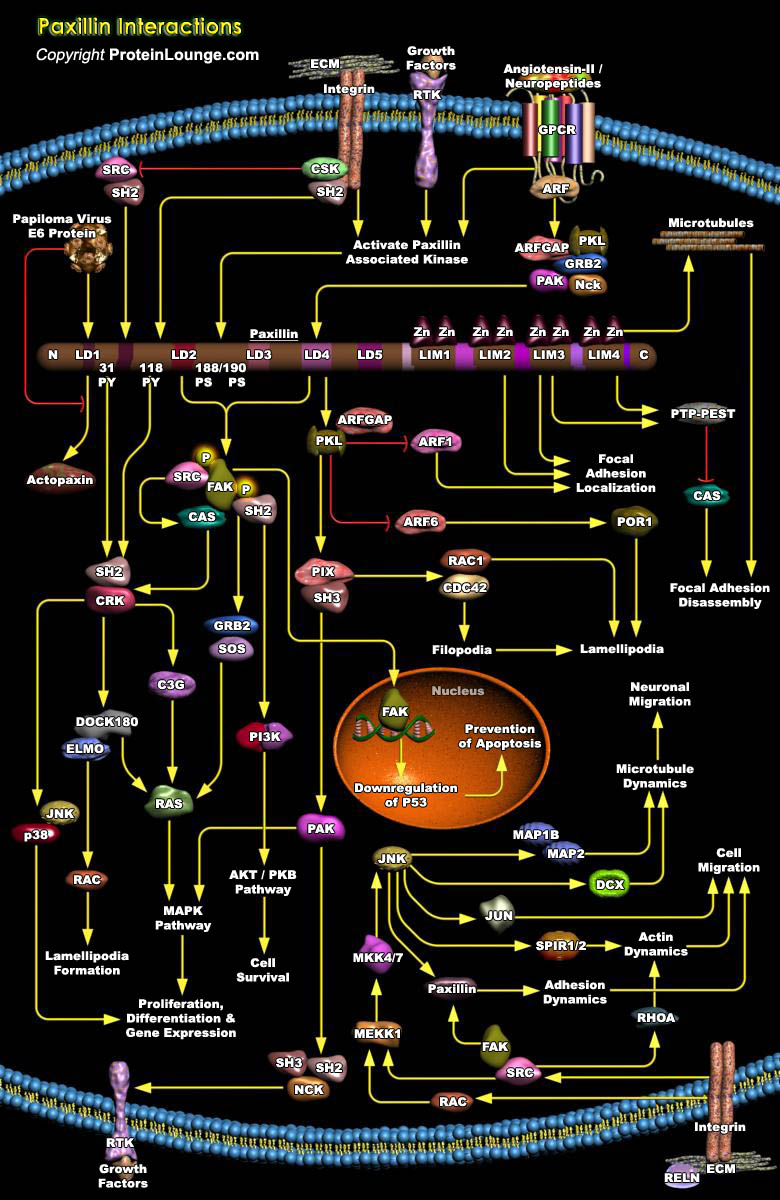
Signals that derive from cell adhesion to the ECM (Extracellular Matrix) regulate important physiological events including cell motility and growth, and most often involve changes in the organization of the actin cytoskeleton. Cells interact with the ECM via transmembrane receptors, termed integrins, located at the cell surface. Binding of integrins to the ECM is accompanied by a localized clustering of these receptors, with the subsequent recruitment of structural and signaling molecules to the sites of matrix attachment, focal contacts, providing links to the actin cytoskeleton (Ref.1). Numerous proteins present at the cytoplasmic face of FA (Focal Adhesions) include cytoskeletal proteins such as vinculin and talin. In addition, numerous "signaling"[..]
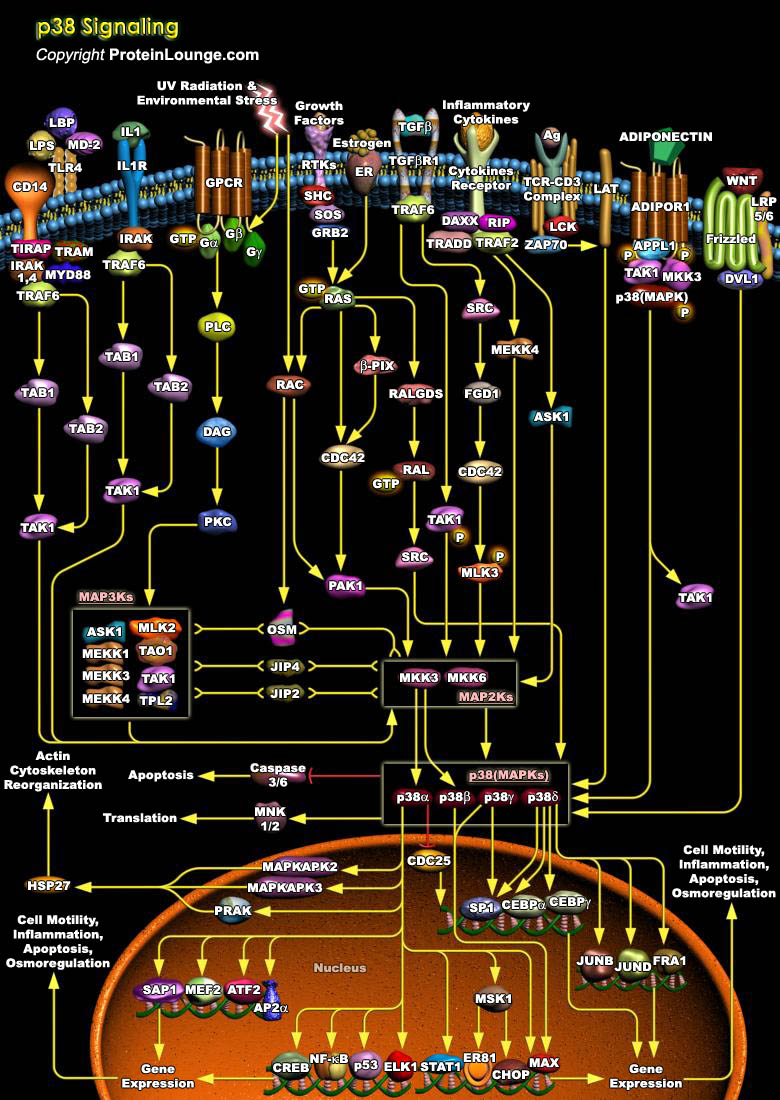
P38s are a class of mitogen-activated protein kinases(MAPKs) are involved in cell differentiation, apoptosis and autophagy MAPKs are a family of Serine/threonine kinases that comprise 3 major subgroups, namely, ERK (Extracellular signal–Regulated Kinase), p38 MAPK and JNK (c-Jun N-terminal Kinases). Despite the diversity in function and upstream signaling events, MAPKs are always activated by a highly conserved mechanism that involves phosphorylation on both a Thr (Threonine) and a Tyr (Tyrosine) residue catalyzed by a MAPK kinase. The phosphorylation motif Thr-Xaa-Tyr is located in the activation loop or T loop whose amino acid sequence varies among different MAPK subfamilies. Accordingly, there are different activating MAPK Kinases that in most cases are[..]
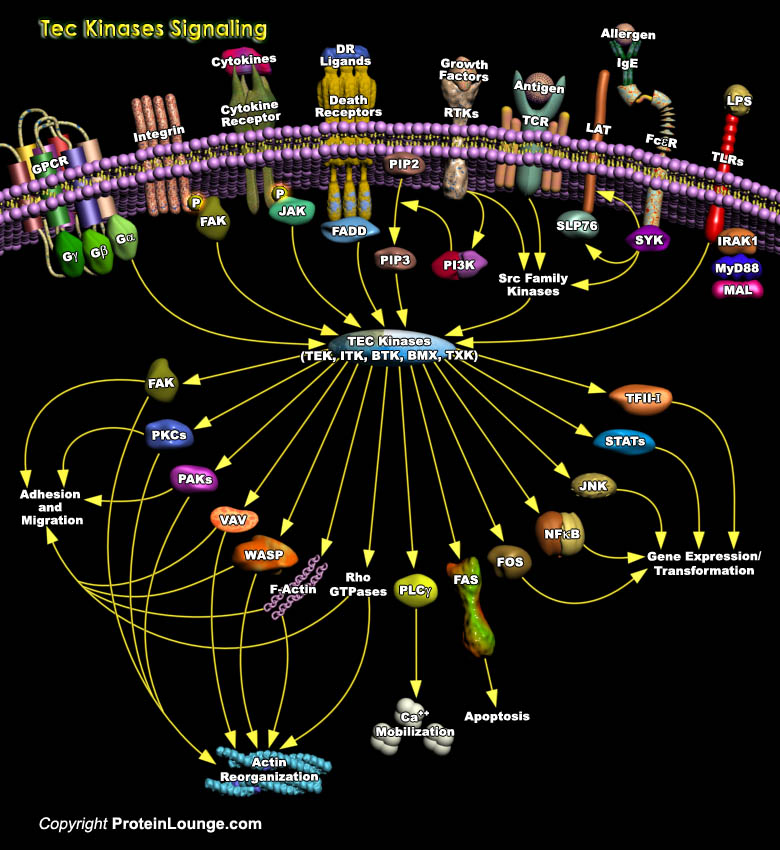
Nonreceptor PTKs (Protein Tyrosine Kinases) are essential for the development and activation of B-Cells and T-Cells. The Tec kinases represent the second largest family of mammalian non-RTKs (Receptor Tyrosine Kinases), which are activated in blood cells by stimulation of Cytokine Receptors, Lymphocyte surface antigens, GPCR (G-Protein Coupled Receptors), receptor type PTKs, or Integrins (Ref.1). Tec family members include Tec, BTK (Bruton’s Tyrosine Kinase), ITK (IL-2-Inducible T-cell Kinase)/ EMT/TSK, BMX (Bone Marrow Kinase)/ETK (Epithelial and Endothelial Tyrosine Kinase) and RLK (Resting Lymphocyte Kinase)/TXK. Expression of most Tec kinases is restricted to hematopoietic[..]
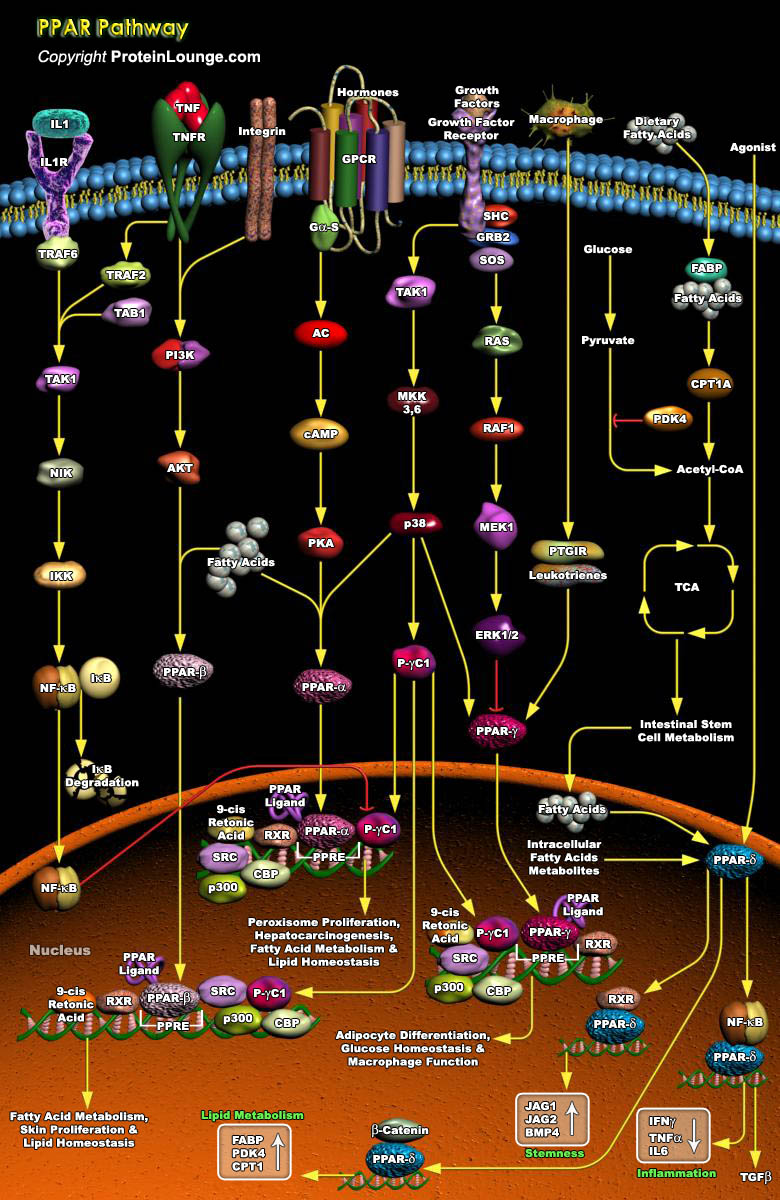
Nuclear hormone receptors are transcription factors that bind DNA and regulate transcription in a ligand-dependent manner. PPARs (Peroxisome Proliferator-Activated Receptors) are ligand-inducible transcription factors that belong to the nuclear hormone receptor superfamily, together with the receptors for thyroid hormone, retinoids, steroid hormones and vitamin D that act as ligand-activated transcription factors. PPARs regulate gene expression by binding with RXR (Retinoid X Receptor) as a heterodimeric partner to specific DNA sequence elements termed PPRE (Peroxisome Proliferator Response Element) (Ref.1). This heterodimeric transcription factor complex then binds to cognate sequences in promoter regions of target genes involved in the catabolism of fatty acids. PPAR[..]
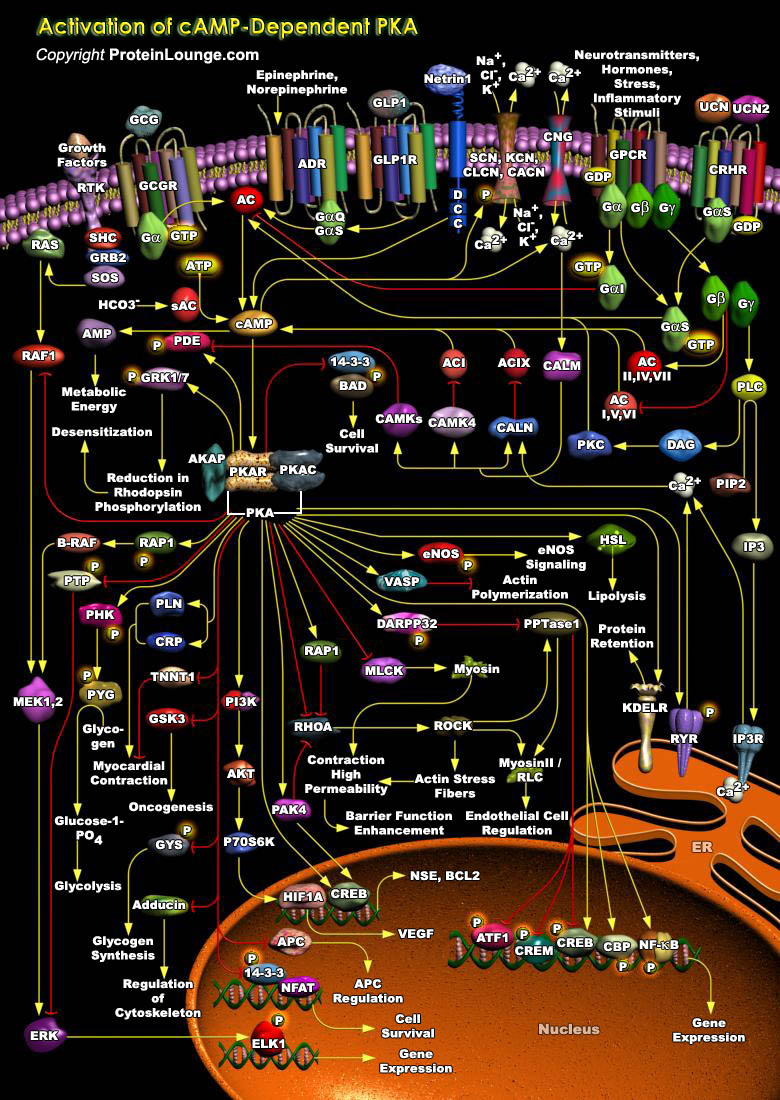
cAMP (Cyclic 3', 5'-Adenosine Monophosphate)-dependent Protein Kinase, commonly known as PKA (Protein Kinase-A), is a second messenger-dependent enzyme that has been implicated in a wide range of cellular processes, including transcription, metabolism, cell cycle progression and apoptosis. Known modulators of PKA activity include factors that either activate or inhibit AC (Adenylate Cyclase), resulting in an increase or decrease in cAMP levels. The enzyme occurs naturally as a four-membered structure with two regulatory (R) and two catalytic (C) subunits. Four genes encode the R subunits (RI-Alpha, RI-Beta, RII-Alpha and RII-Beta), and three encode the C subunits (C-Alpha, C-Beta and C-Gamma). Although there are major differences in the tissue distribution, biochemical[..]
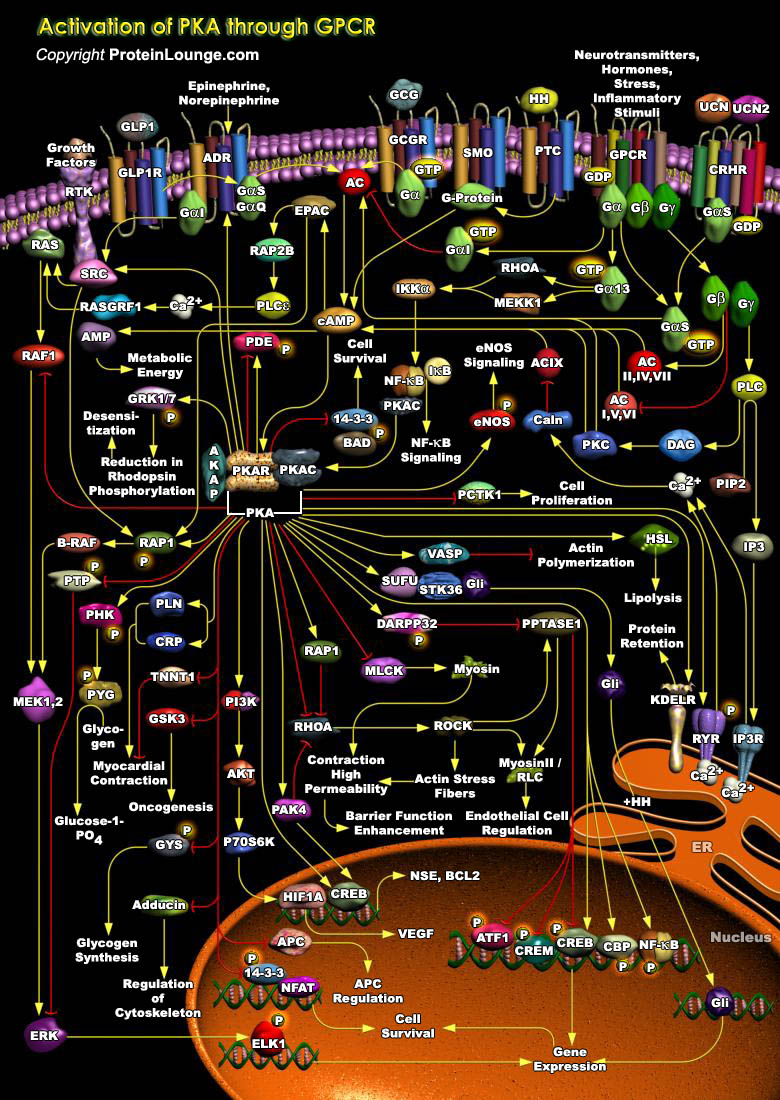
PKA (Protein Kinase-A) is a second messenger-dependent enzyme that has been implicated in a wide range of cellular processes, including transcription, metabolism, cell cycle progression and apoptosis. Known modulators of PKA activity include factors that either activate or inhibit AC (Adenylate Cyclase), resulting in an increase or decrease in cAMP (Cyclic Adenosine 3',5'-monophosphate) levels. The enzyme occurs naturally as a four-membered structure with two regulatory (R) and two catalytic (C) subunits. Four genes encode the R subunits (RI-Alpha, RI-Beta, RII-Alpha and RII-Beta), and three encode the C subunits (C-Alpha, C-Beta and C-Gamma). Although there are major differences in the tissue distribution, biochemical and physical properties of the R subunit isoforms,[..]
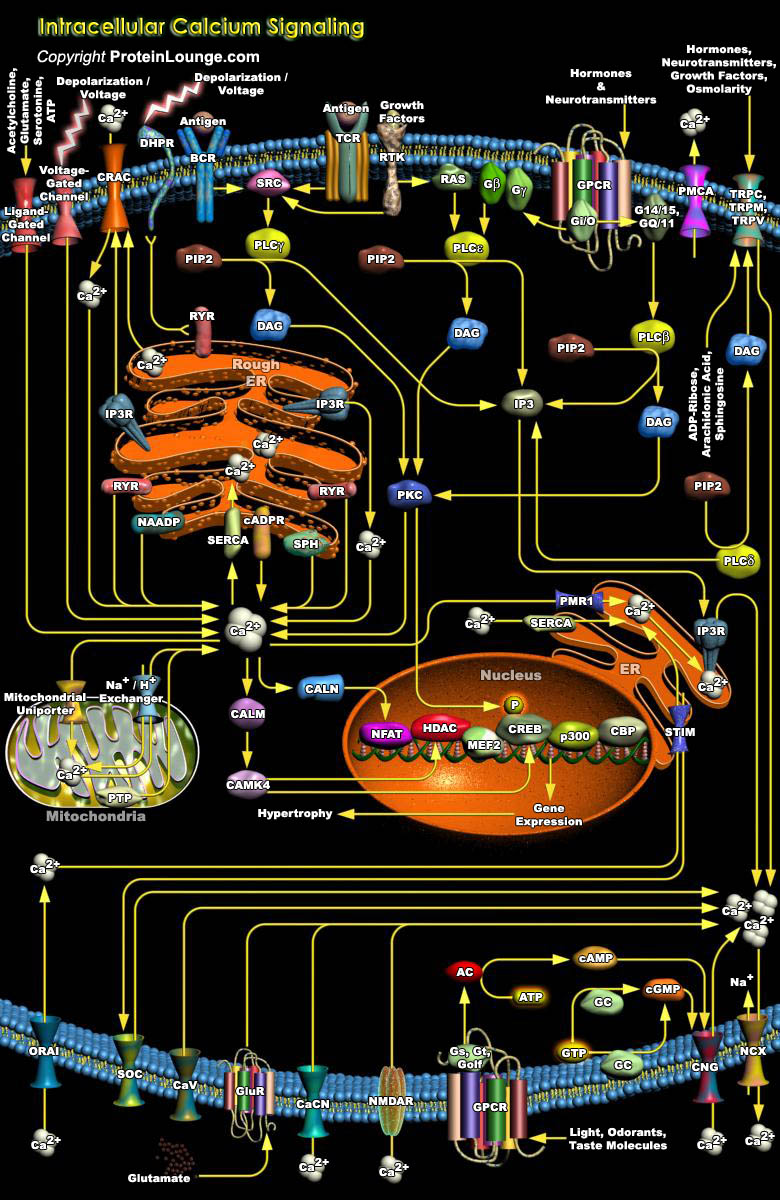
Despite tremendous diversities in their expression, cellular activities in virtually all cell types are regulated by common intracellular signaling systems, and calcium is one important ubiquitous intracellular messenger, controlling a diverse range of cellular processes, such as gene transcription, muscle contraction and cell proliferation. In response to adequate stimuli, [Ca2+]i (Intracellular Ca2+ concentration) increases, oscillates and decreases, leading to the activation, modulation and termination of cell function. Numerous channels and pumps allow this particular cation to enter and exit cells and move between the cytosol and intracellular stores(Ref.1). When calcium signaling is stimulated in a cell, Ca2+ enters the cytoplasm from one[..]

