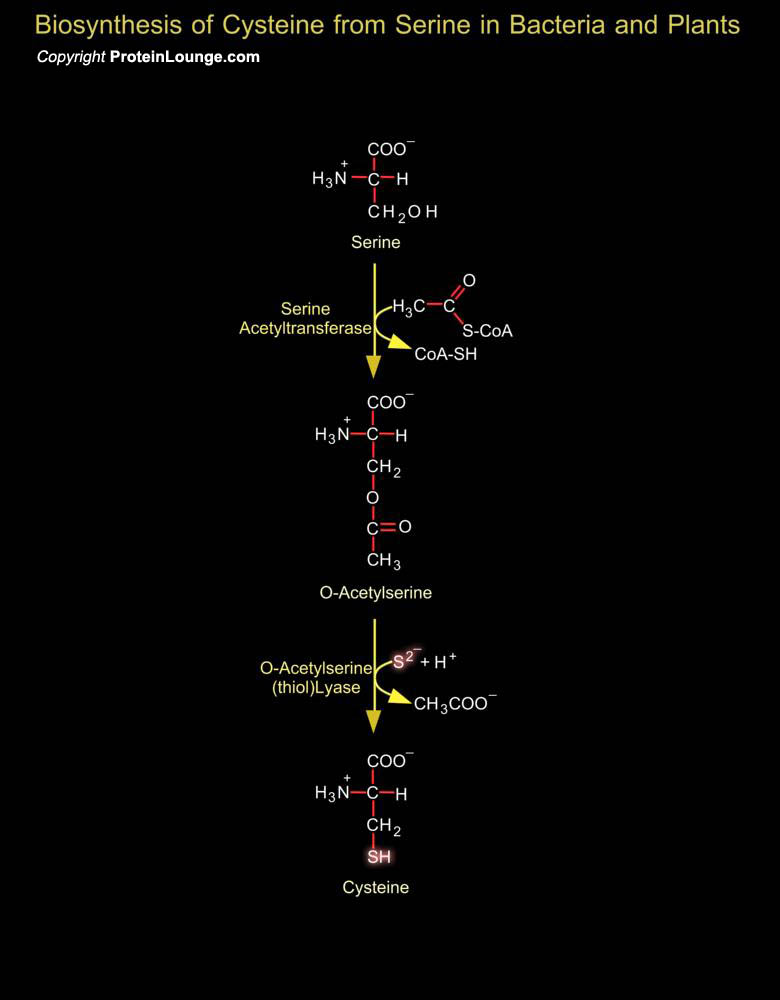
Inorganic sulfur in the environment (primarily sulfate, but also sulfur, and sulfite) must undergo fixation to be utilized by organisms. The fixation of sulfate is largely confined to plants and bacteria and biosynthesis of cysteine represents the final step of sulfate assimilation in these organisms. Fixation begins with the formation of PAPS (3'-Phosphoadenosine-5'-Phosphosulfate). PAPS is an activated sulfate compound and an intermediate in all organisms for sulfate esterification, such as the synthesis of chondroitin sulfate. It is formed in a two-step reaction from sulfate ion and two molecules of ATP. In plants, the main pathway of sulfate reduction is via APS (Adenosine-5'-Phosphosulfate) rather than PAPS (i.e. APS can be utilized directly, without[..]
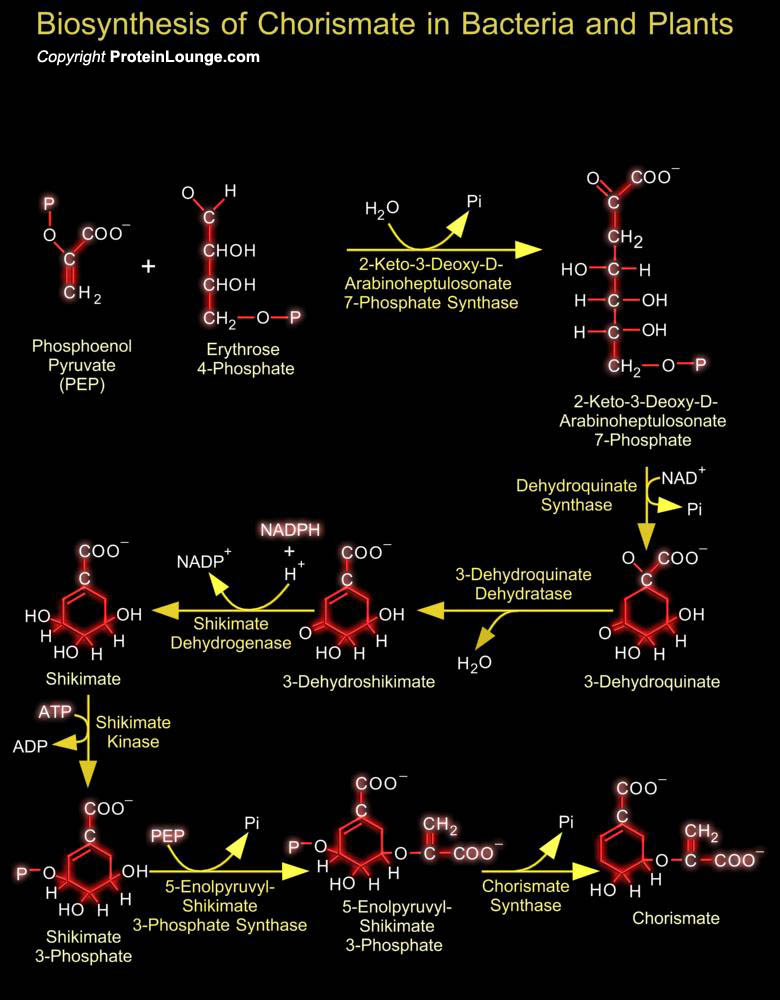
In micro-organisms and plants the biosynthesis of aromatic compounds proceeds via the common seven-step aromatic or shikimate pathway to the branch point intermediate chorismate. This intermediate is subsequently converted to the three aromatic amino acids via specific terminal pathways. Many other aromatic compounds are derived either partially or entirely from chorismate or from other pathway intermediates or end products.The first step in the biosynthesis of Chorismate involves PEP (Phosphoenolpyruvate) from glycolysis and E-4-P (Erythrose 4-Phosphate) from the Pentose Phosphate Cycle. These two precursors are condensed and then cyclized to form 3-Dehydroquinate, followed by removal of a water and a reduction step to produce Shikimate. The first cyclic intermediate[..]
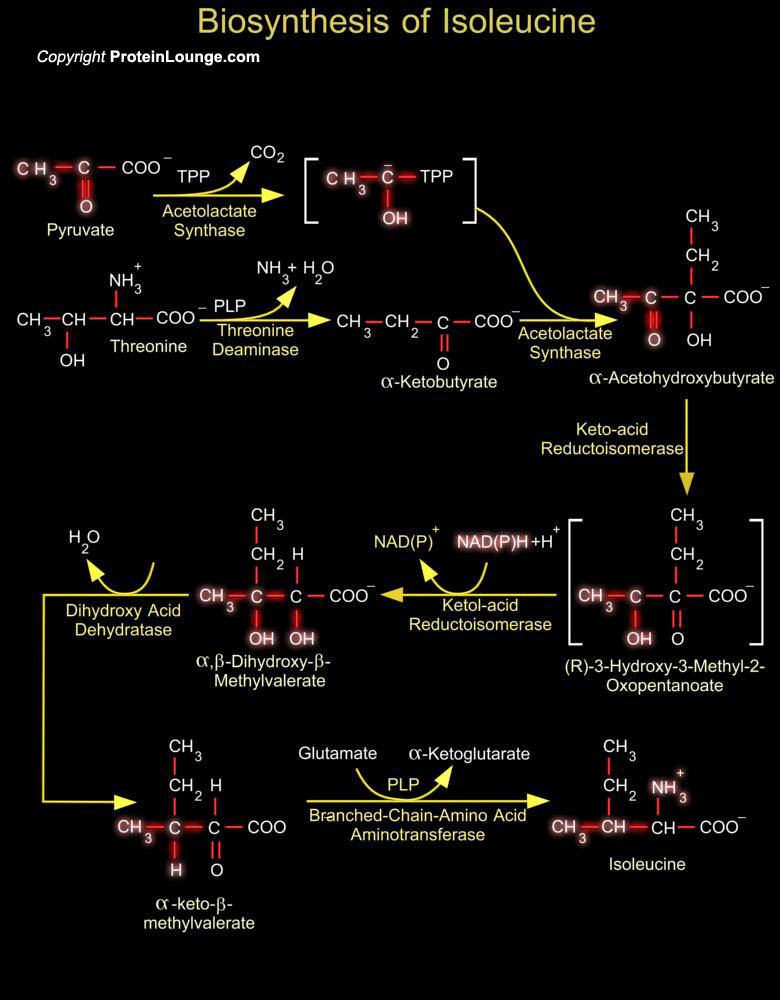
Isoleucine encoded by the codons AUU, AUC, and AUA used in the biosynthesis of proteins. It is a α-amino acid that contains an α-amino group, an α-carboxylic acid group, and a hydrocarbon side chain. It is classified as a non-polar, uncharged (at physiological pH), branched-chain, aliphatic amino acid. Isoleucine is essential in humans, meaning the body cannot synthesize it, and must be ingested in our diet. Isoleucine is synthesized from pyruvate employing leucine biosynthesis enzymes in other organisms such as bacteria (Ref.1).The biosynthesis pathway of L-valine and L-isoleucine from L-threonine is a part of the super pathway of branched amino acid biosynthesis that also generates L-leucine. The first enzyme involved in the pathways[..]
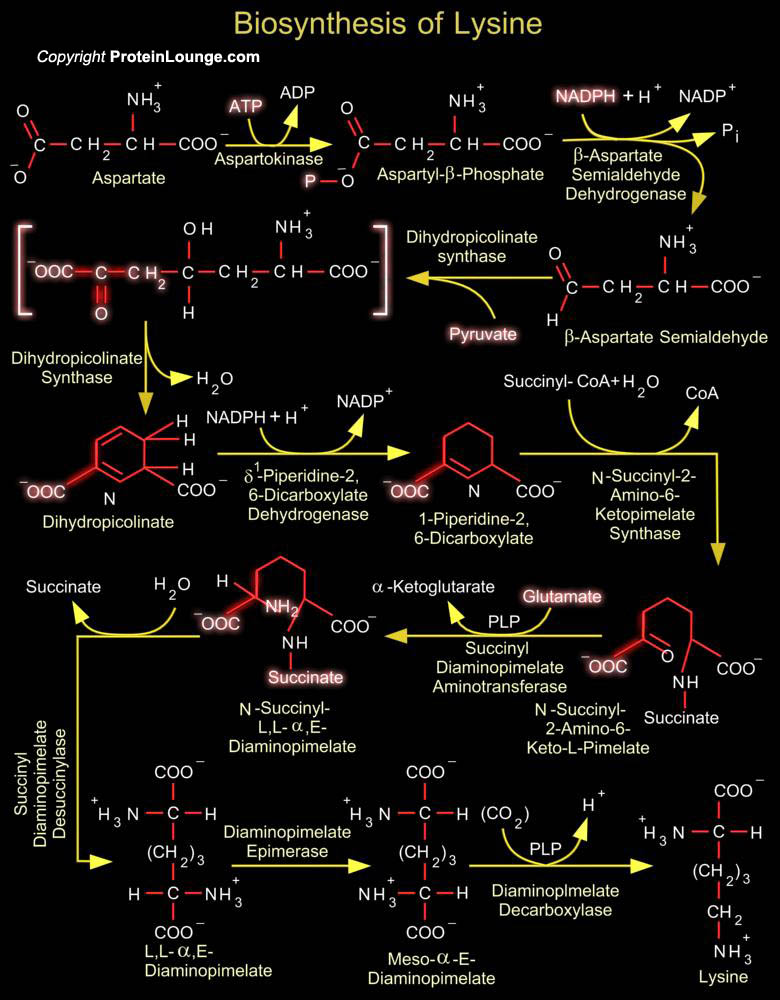
The amino acid L-lysine is synthesized by plants and microorganisms by two different pathways, one proceeding via Diaminopimelate and the other via Alpha-Aminoadipate. Humans, however, cannot synthesise the compound and so it is an essential part of their diet. The Diaminopimelate pathway operates in bacteria, lower fungi and green plants. This pathway is the source of the lysine and Diaminopimelate incorporated into bacterial cell wall peptidoglycan and therefore there has been extensive investigation of its enzymes with a view to the development of new antibacterial agents. In Euglenoids and higher fungi lysine biosynthesis occurs via the Homocitrate-Aminoadipate pathway. An intermediate on this pathway (Alpha-Aminoadipic acid) is necessary for Beta-lactam antibiotic[..]
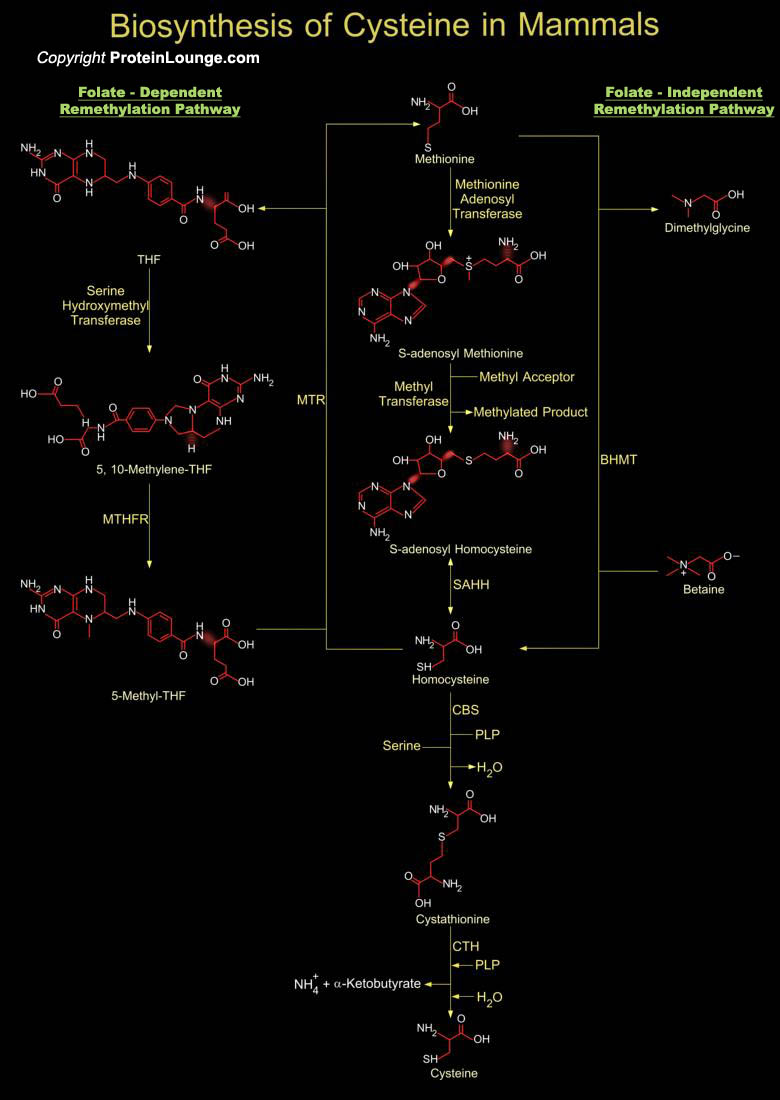
Cysteine, a sulfur-containing amino acid, is indispensable for the survival of virtually all living organisms, from bacteria to higher eukaryotes. This amino acid is implicated in several processes, including the stability, structure, regulation of catalytic activity, and post-translational modification of various proteins. Due to the ability of its thiol group to undergo redox reactions, Cysteine forms the basic building block of all thiol antioxidants, acting as a direct antioxidant and also as a precursor for the biosynthesis of glutathione, trypanothione, or ovothiol. In addition, cysteine is also essential for the synthesis of biomolecules, including coenzyme A, hypotaurine, taurine, and ubiquitous iron-sulphur (Fe-S) clusters, which are involved in electron[..]
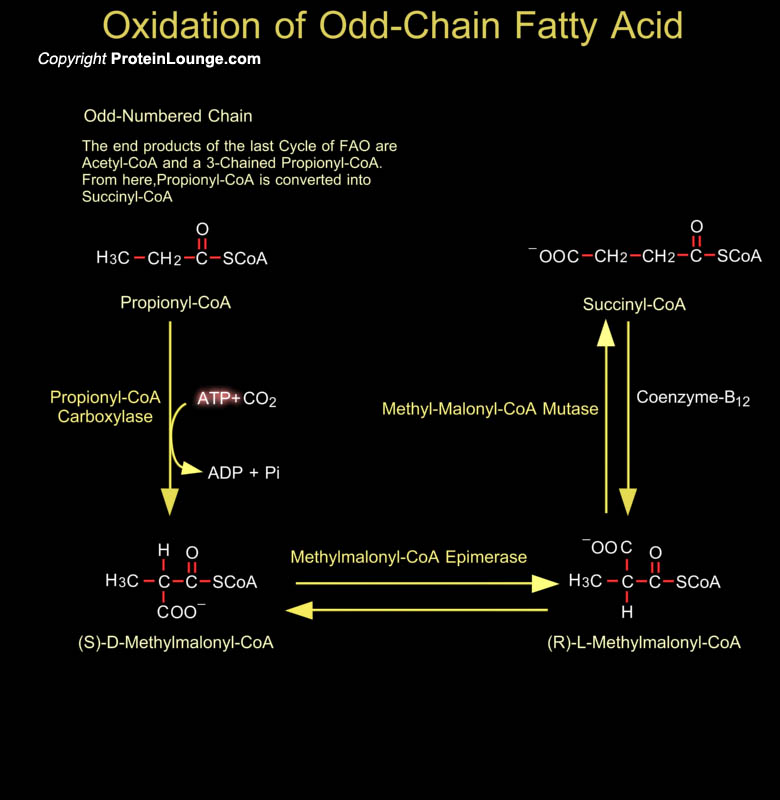
Most fatty acids have even number of carbon atoms and are therefore completely converted to Acetyl-CoA. Some plants and marine organisms, however, synthesize fatty acids with an odd number of carbon atoms. The final round of Beta-Oxidation of these fatty acids forms Propionyl-CoA, which is converted to Succinyl-CoA for entry into the Citric Acid Cycle. Propionyl-CoA can be derived from breakdown of certain amino acids (Isoleucine, Valine and Methionine), but in mammalian cells these pathways exist in mitochondria. Bacteria in the ruminant animal digestive system provide a source of propionate which eventually gets incorporated as odd-numbered fatty acids in milk fats, and this is the major source for human metabolism. The conversion of[..]
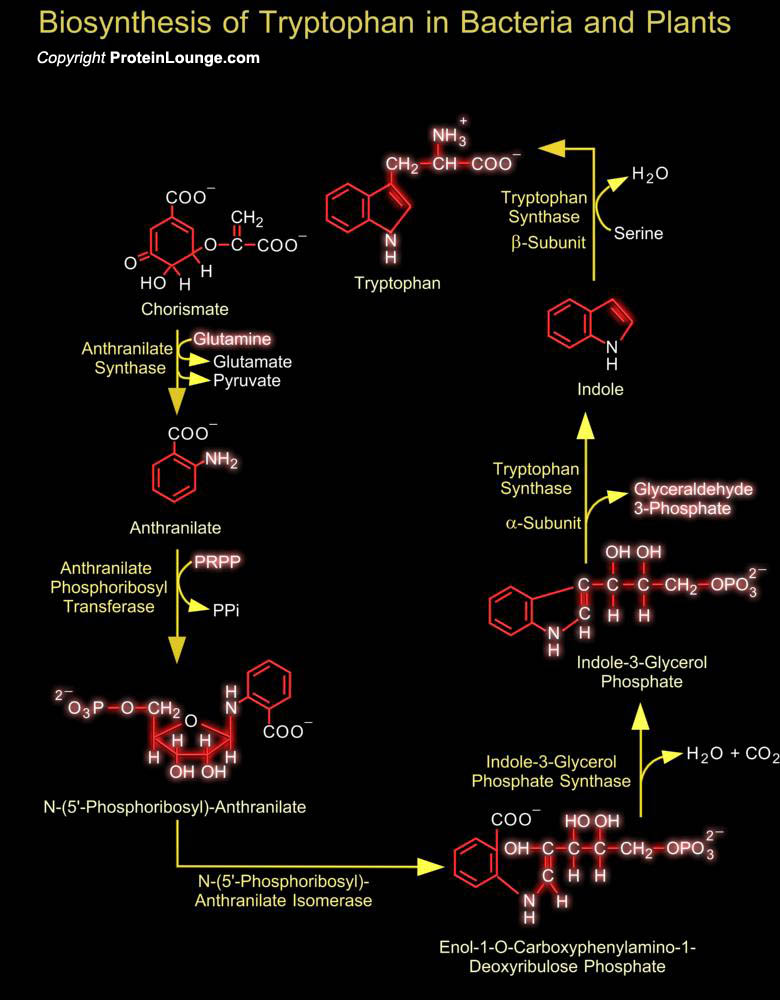
Tryptophan is an amino acid essential to survival. Amino acids serve as building blocks for proteins, as well as serving as starting points for the synthesis of vitamins and many other crucial cellular molecules. While most plants and microorganisms can produce all the amino acids they need, tryptophan is one of eight amino acids that cannot be produced by animals. Humans and animals do not themselves have the biosynthetic machinery to synthesize tryptophan but rely on dietary intake from bacteria and plants that do produce it. Tryptophan is the least abundant of the essential amino acids. However, it is also one of the most crucial, as it is involved not only in protein synthesis, but also in the formulation of niacin and the neurotransmitter serotonin. Serotonin is[..]
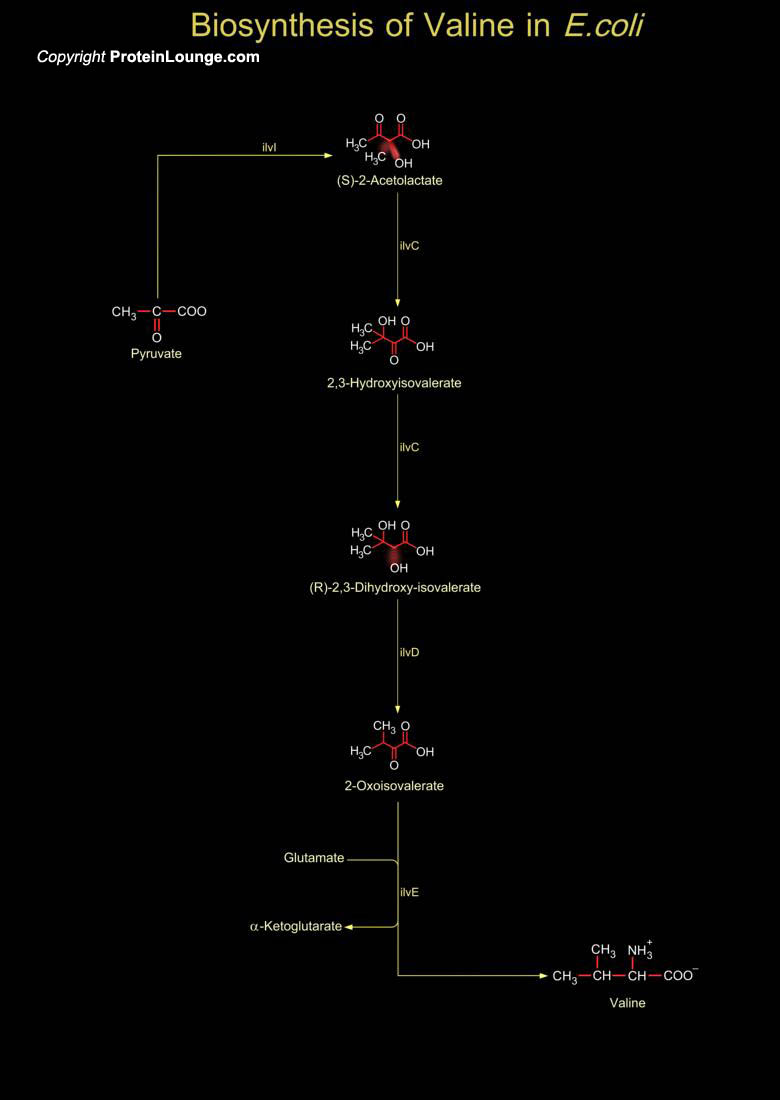
Valine is a hydrophobic amino acid, it often forms the helical structures within interior proteins. Valine is an essential amino acids and must be obtained in the diet in human but not synthesized in human. It is generally biosynthesized by plants, algae, fungi, bacteria and archaea. Important sources of valine include soy flour, cottage cheese, fish, meats, and vegetables. Valine is incorporated into proteins and enzymes at the molar rate of 6.9 percent when compared to the other amino acids. In Escherichia coli (E.coli), Valine is biosynthesized from two Pyruvate molecules where two molecules of Pyruvate are converted to Alpha-Acetolactate by the enzyme ilvI (acetolactate synthase/acetohydroxybutanoate synthase, catalytic subunit) yielding a molecule of CO2.[..]
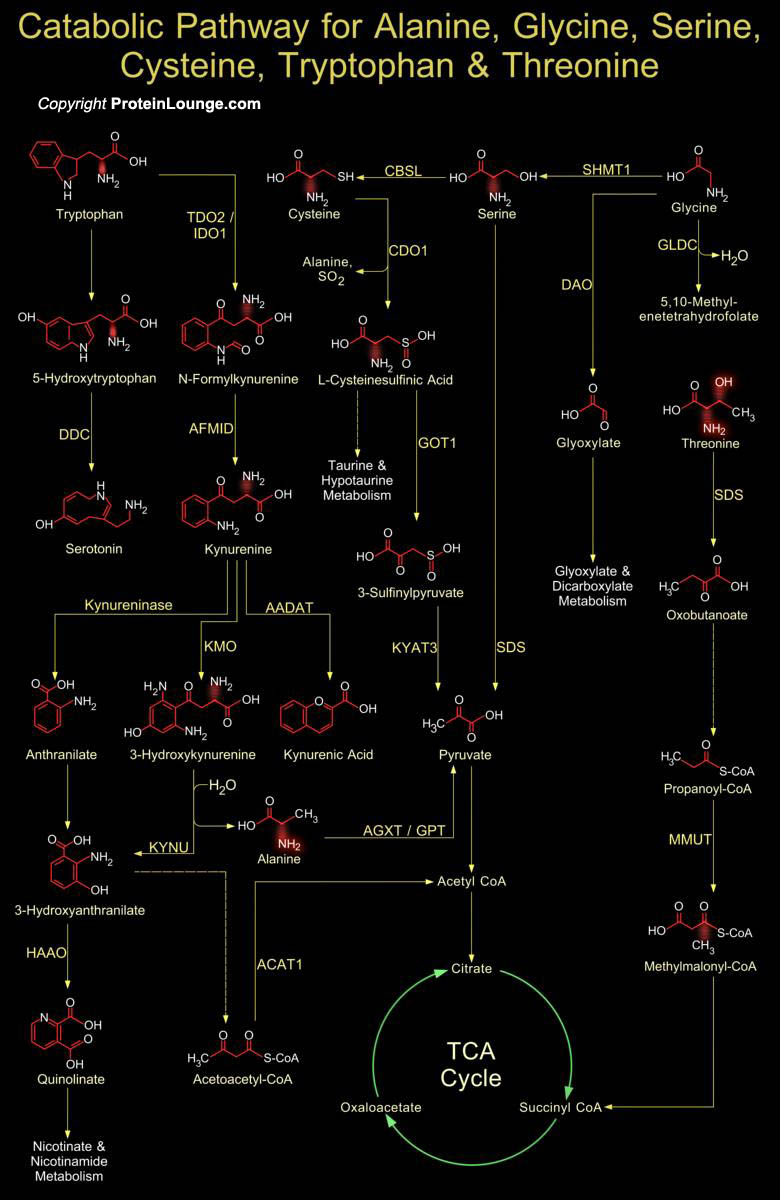
Five amino acids, Alanine, Cysteine, Glycine, Serine and Threonine are broken down to yield Pyruvate. Tryptophan is included in this group since one of its breakdown products is Alanine, which is transaminated to Pyruvate. Alanine is an important in inter tissue nitrogen transporter and is an important part of the Glucose-Alanine cycle. The alanine catabolic pathway involves a simple aminotransferase reaction that directly produces pyruvate. The transamination is carried out by GPT (Glutamic--Pyruvic Transaminase). Post pyruvate production, based on metabolic requirements of the cell, pyruvate can either be oxidized by the PDH complex to acetyl-CoA and diverted into the TCA cycle or it can be diverted into the gluconeogenic pathway to release glucose[..]
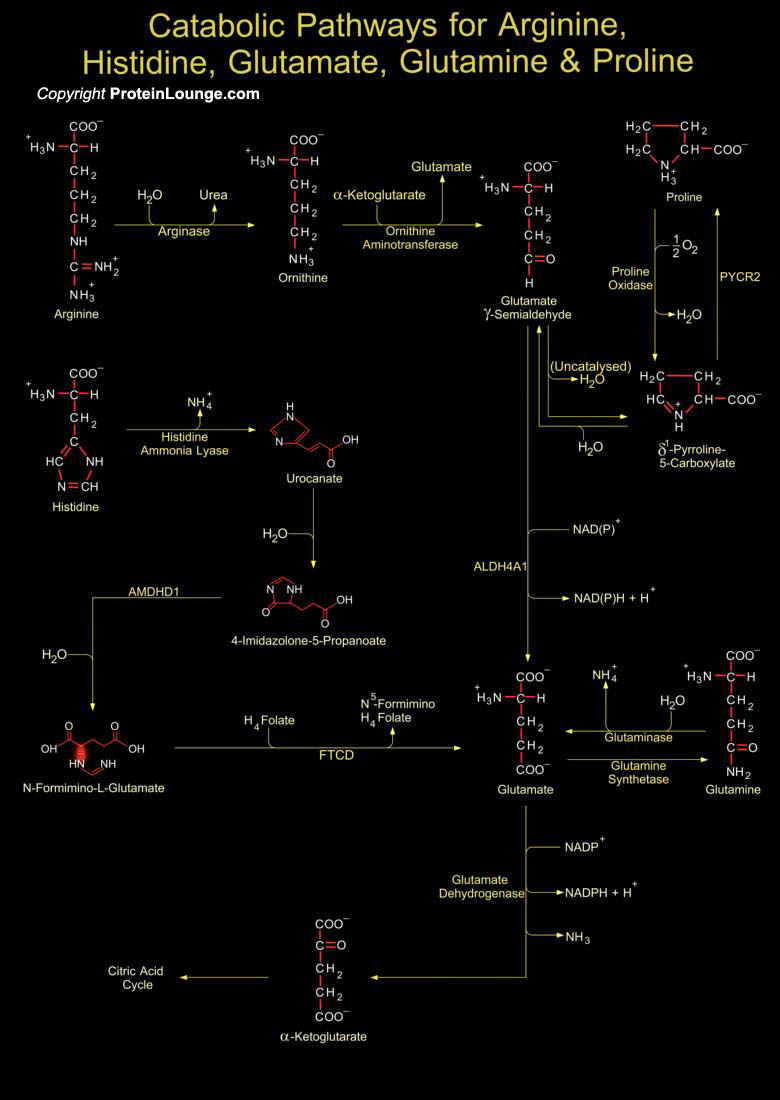
Arginine serves as a precursor for synthesis of protein, nitric oxide, creatine, polyamines, agmatine, and urea. It is synthesized from glutamine, glutamate, and proline and is metabolically interconvertible with the amino acids proline and glutamate. ARG1 (arginase 1) converts L-arginine into L-ornithine and urea. L-ornithine is acted upon by OAT (ornithine aminotransferase) to further synthesize L-glutamate-5-semialdehyde which spontaneously undergoes non-enzymatic reaction to produce P5C (δ 1-pyrroline-5-carboxylate). P5C can further initiate proline biosynthesis by the enzyme PYCR2 (pyrroline-5-carboxylate reductase 2) (Ref.1, 2&3). Glutamate an important neurotransmitter serves as a precursor for the biosynthesis of amino[..]
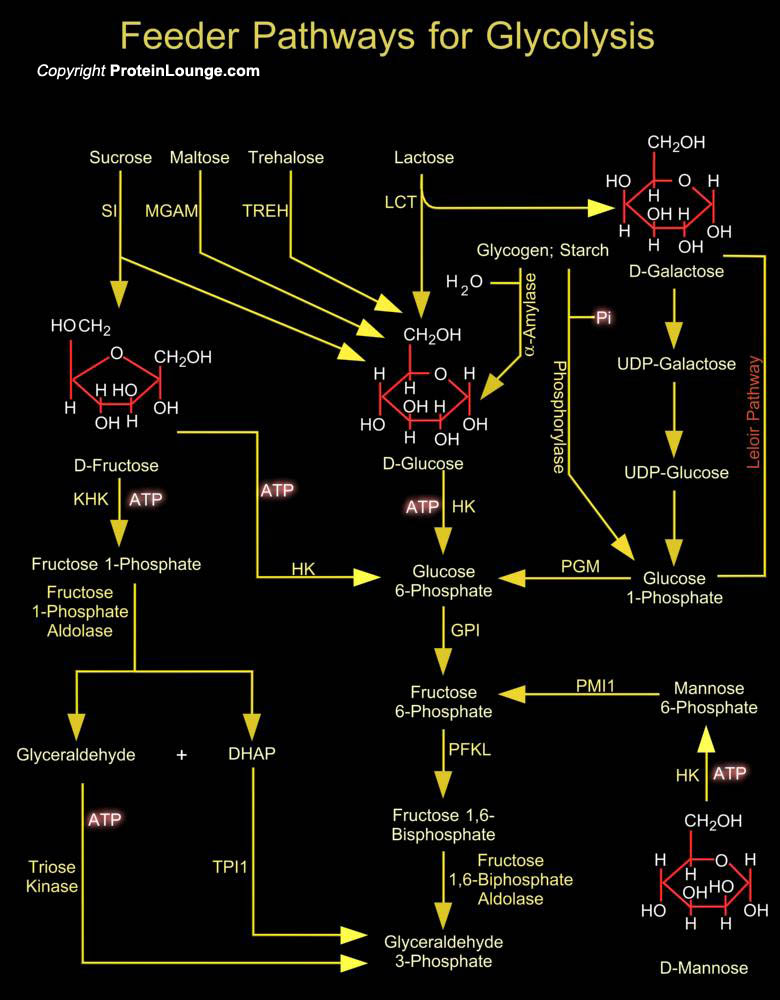
Many carbohydrates besides Glucose (or D-Glucose) meet their catabolic fate in Glycolysis, after being transformed into one of the Glycolytic intermediates. The most significant are the storage polysaccharides Glycogen and Starch; the disaccharides Maltose, Lactose, Trehalose and Sucrose; and the monosaccharides Fructose (or D-Fructose), Mannose (or D-Mannose) and Galactose (or D-Galactose) (Ref.1). Glycogen in animal tissues and in microorganisms; and Starch in plants, are mobilized for use within the same cell by a phosphorolytic reaction catalyzed by Phosphorylase (that is Glycogen Phosphorylase in animals and in microorganisms or Starch Phosphorylase in plants). These enzymes catalyze an attack by Pi (Inorganic Phosphate) on the (Alpha1-4) glycosidic linkage[..]
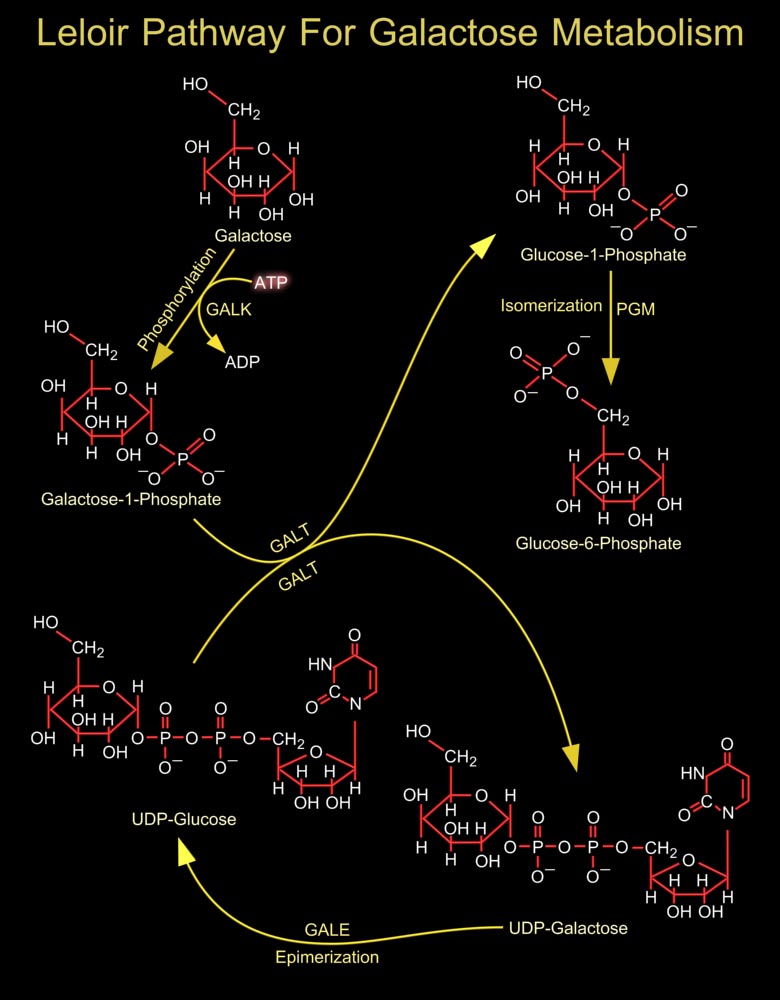
In most organisms, the conversion of Galactose to the more metabolically useful Glucose-1-Phosphate is accomplished by the action of four enzymes that constitute the Leloir pathway. In the first step of this pathway, Beta-D-Galactose is epimerized to Alpha-D-Galactose by GALM (Galactose Mutarotase/Aldose 1-Epimerase) (Ref.1). The active site of GALM is positioned in a rather open cleft with the hydroxyl groups of Galactose lying within hydrogen bonding distance to a number of side chains, the reaction catalyzed by human GALM proceeds via Glu-307 (Glutamic acid-307) and His-176 (Histidine-176). Till now no diseases have been attributed to mutations in human GALM. The next step involves the ATP-dependent phosphorylation of Alpha-D-Galactose by GalK (Galactokinase) to[..]

