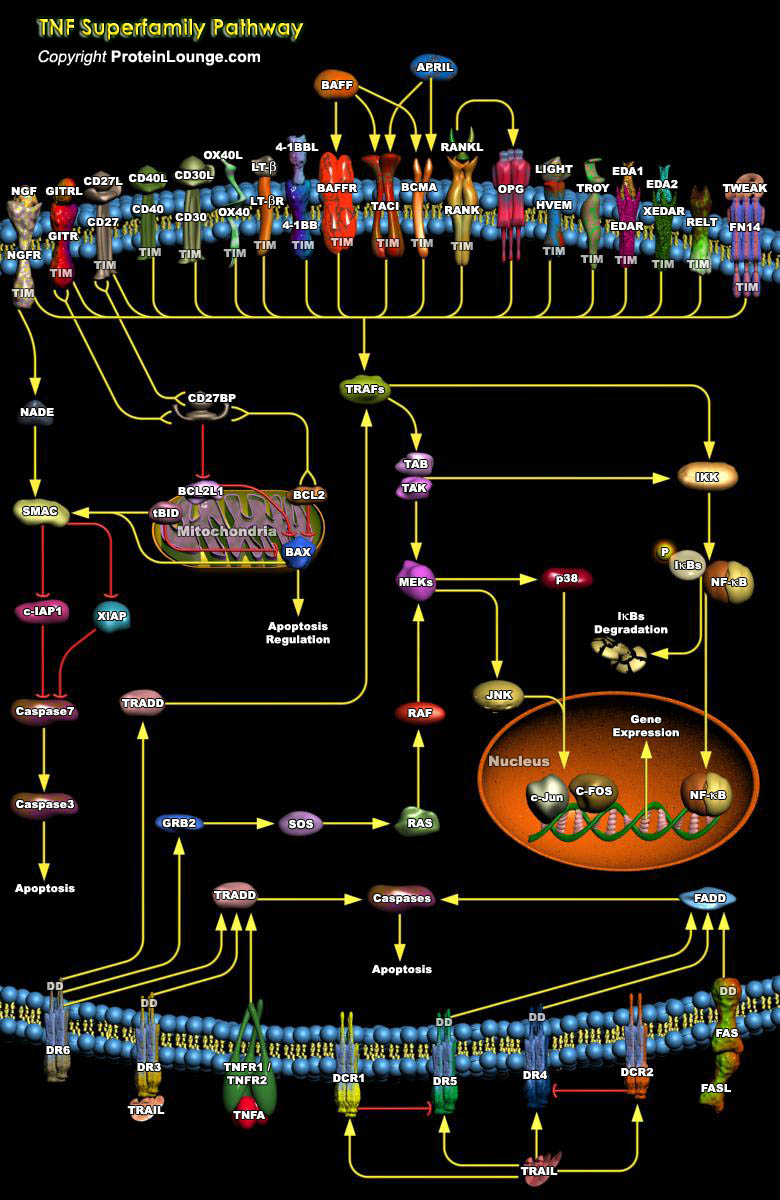
Tumor necrosis factor (TNF) superfamily proteins consisting of 19 members and 29 receptors regulate cell proliferation, cell death, and morphogenesis. The tumor necrosis factor superfamilies members provide key communication signals between various cell types during development, especially in the skin, bones, and lymphoid organs, and maintain organ homeostasis and initiate tissue responses. Because of these essential roles, TNFRSFs and the TNF superfamily (TNFSF) ligands are attractive targets for treating diverse diseases, such as autoimmunity, cancer, infectious diseases, and graft-versus-host disease (Ref.1 and 2). The TNF-related ligands assemble into trimers that form a highly efficient receptor-clustering and signal-initiating mechanism. TNF receptors share a[..]
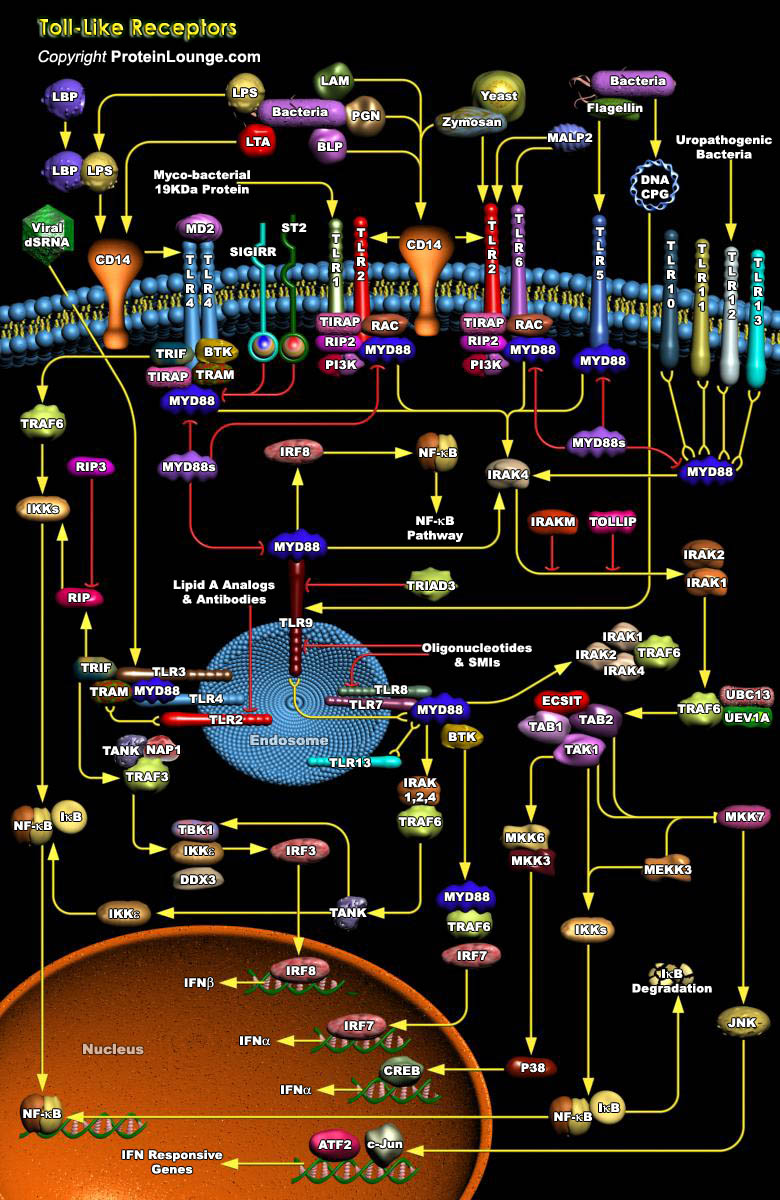
TLRs (Toll-like receptors) are transmembrane proteins expressed by cells of the innate immune system, which recognize invading microbes and activate signaling pathways that launch immune and inflammatory responses to destroy the invaders. Toll receptors were first identified in Drosophila. In mammals, the TLR family includes eleven proteins (TLR1-TLR11). Recently, two new members, TLR12 and TLR13 have been discovered in mouse, but not much information is known about them. Mammalian TLRs consist of an extracellular portion containing Leucine-rich repeats, a transmembrane region and a cytoplasmic tail, called the TIR (Toll-IL-1R (Interleukin-1-Receptor)) homology domain. Different TLRs serve as receptors for diverse ligands including Bacterial cell wall components, viral[..]
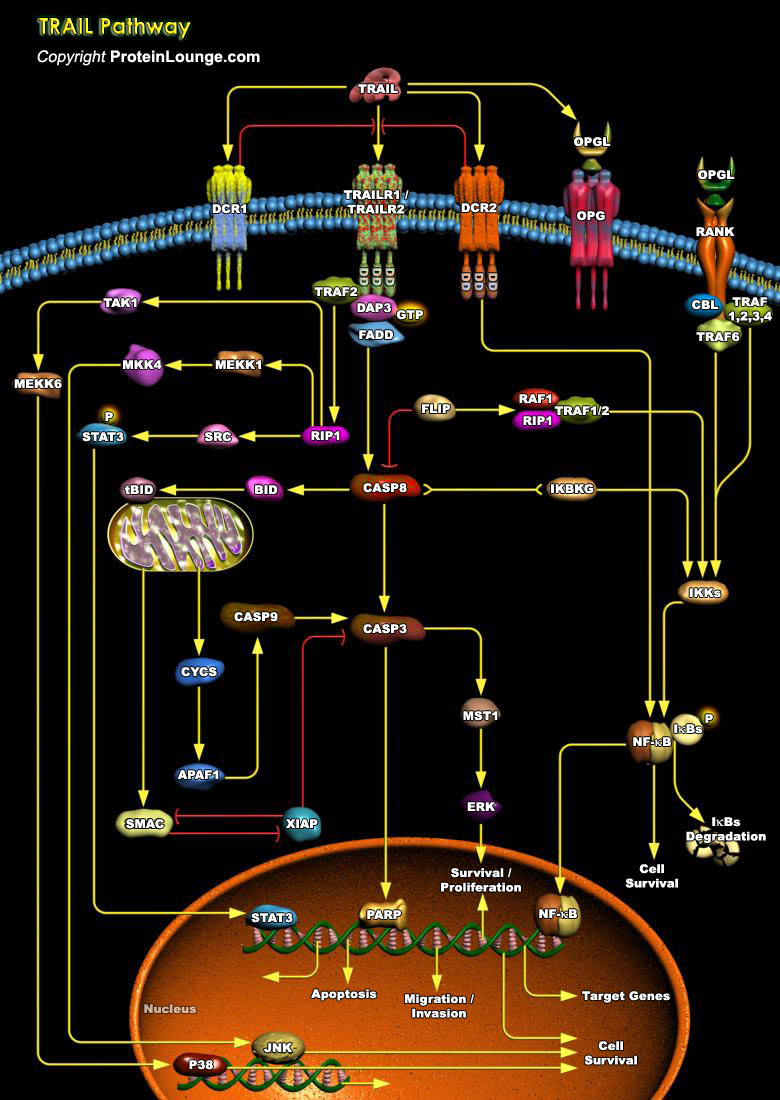
TRAIL (TNF-Related Apoptosis-Inducing Ligand) is a protein consisting of 281 amino acids. It is also called Apo2L. Five proteins, TRAILR1 (DR4), TRAILR2 (DR5/ TRICK2 or KILLER), TRAILR3 (DcR1/ TRID or LIT), TRAILR4 (DcR2 or TRUNDD), and Opg (Osteoprotegerin), have been identified as TRAIL receptors. Both TRAILR1 and TRAILR2 contain the functional DD (Death Domain), capable of inducing apoptosis. The other three receptors DcR1, DcR2 and Opg serve as "decoy" receptors. These three receptors can bind to TRAIL, but cannot induce apoptosis. DcR1 is a glycosylphosphatidylinositol-anchored cell surface protein, which contains the TRAIL-binding region as well as a region that anchors the receptor to the membrane. But unlike the other receptors, DcR1 lacks an[..]
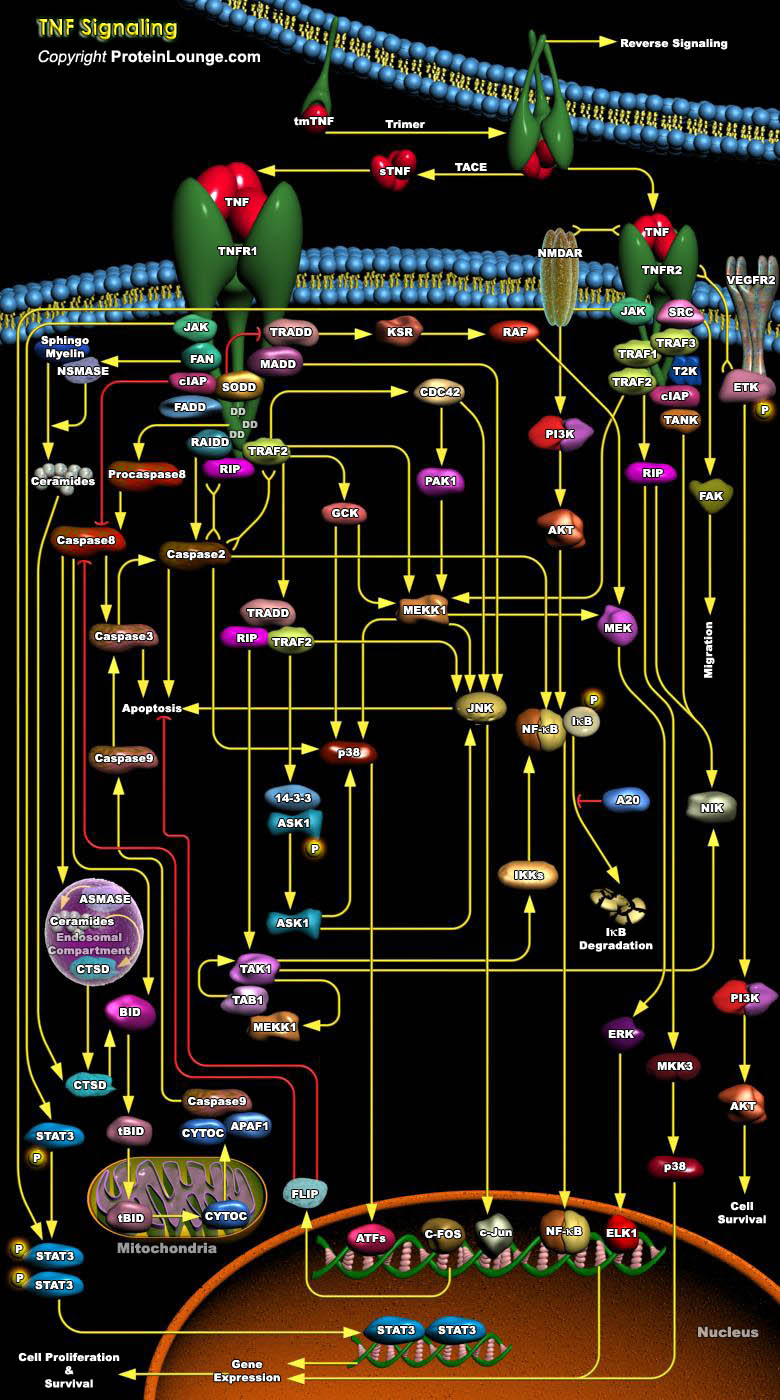
Tumor necrosis factor (TNF) is a pro-inflammatory cytokine with the capacity to induce apoptosis. It is enriched in the tumor microenvironment, promotes tumor growth and subverts innate immune responses to cancer cells. TNF is the best studied member of the TNF superfamily. TNF-alpha can bind to two related receptors, TNF receptors 1 and 2 (TNFR1 and TNFR2), which are also used by other, similar ligands. By binding to TNFR1 and TNFR2, TNF activates distinct signaling pathways important for cell proliferation, cell death and immune responses (Ref.1 and 2). TNFR1 is constitutively expressed in most cell types, whereas TNFR2 is typically restricted to certain subpopulations of immune cells such as CD4+ or CD8+ T cells and a few other cell types such as oligodendrocytes[..]
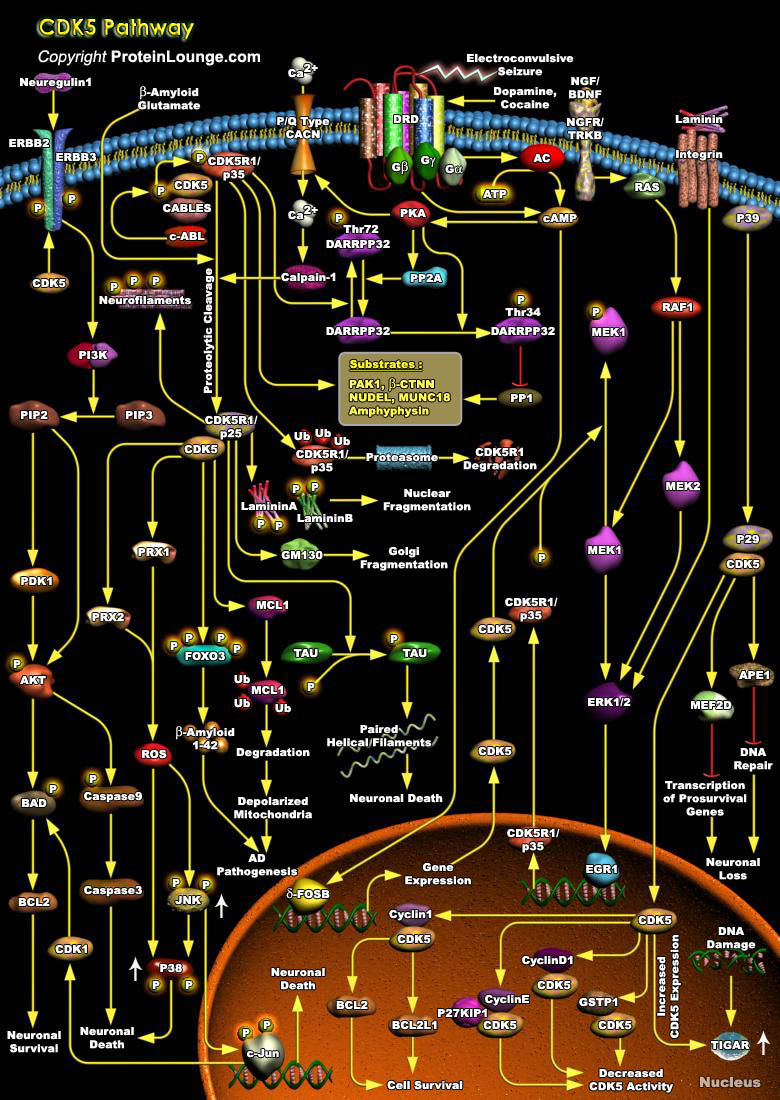
CDKs (Cyclin-dependent kinases) are a group of serine/threonine protein kinases activated by binding to a regulatory subunit cyclin.CDK5(Cyclin dependent kinase-5), a family member of the cyclin-dependent kinases, plays a pivotal role in the central nervous system. During embryogenesis, CDK5 is indispensable for brain development and, in the adult brain, it is essential for numerous neuronal processes, including higher cognitive functions such as learning and memory formation. However, CDK5 activity becomes deregulated in several neurological disorders, such as Alzheimer's disease, Parkinson's disease and Huntington's disease, which leads to neurotoxicity. Therefore, precise control over CDK5 activity is essential for its physiological functions. For its activation[..]
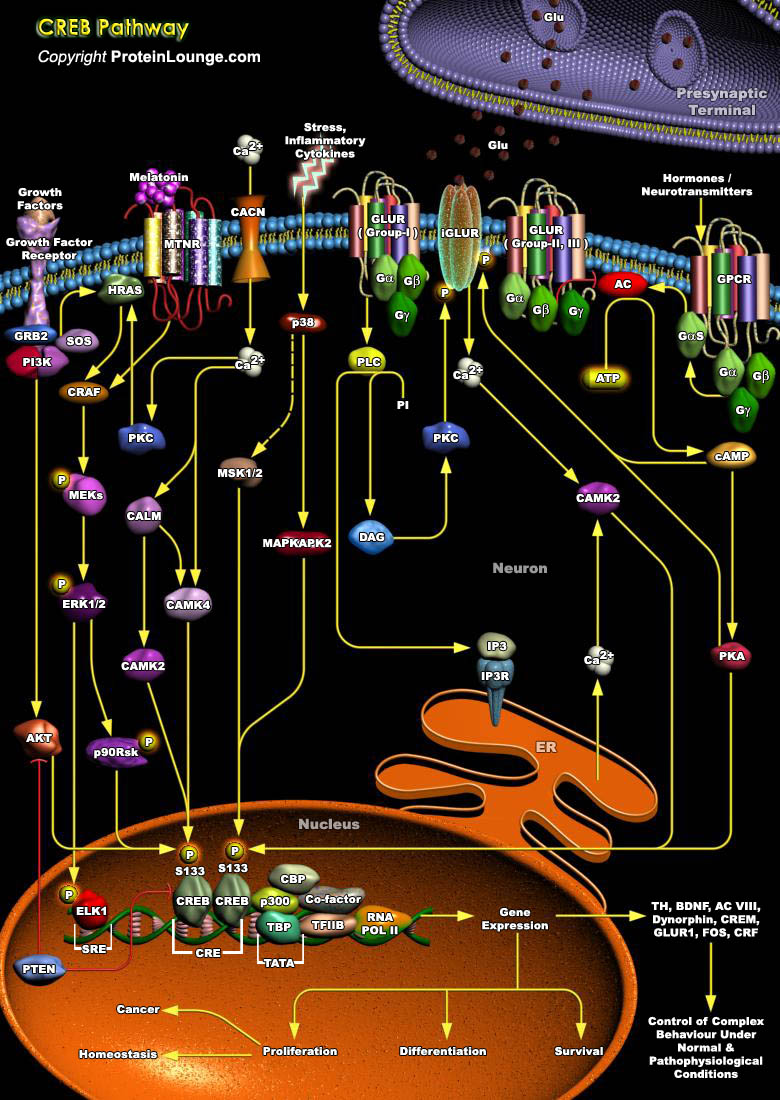
The process of consolidating a new memory and the dynamic complexity of information processing within neuronal networks is greatly increased by activity-dependent changes in gene expression within individual neurons. A leading paradigm of such regulation is the activation of the nuclear transcription factor CREB (cAMP Responsive Element Binding Protein), and its family members the ATF (Activating Transcription Factor) and CREM (cAMP Response Element Modulator), which belong to bZIP (basic/leucine zipper) class of transcription factors that functions in vivo to regulate the proliferation of pituitary cells and thymocytes. Proteins belonging to this class are characterized by the ability to bind to the consensus sequence TGACGTCA (Ref.1, 2 & 3) and contain a leucine[..]
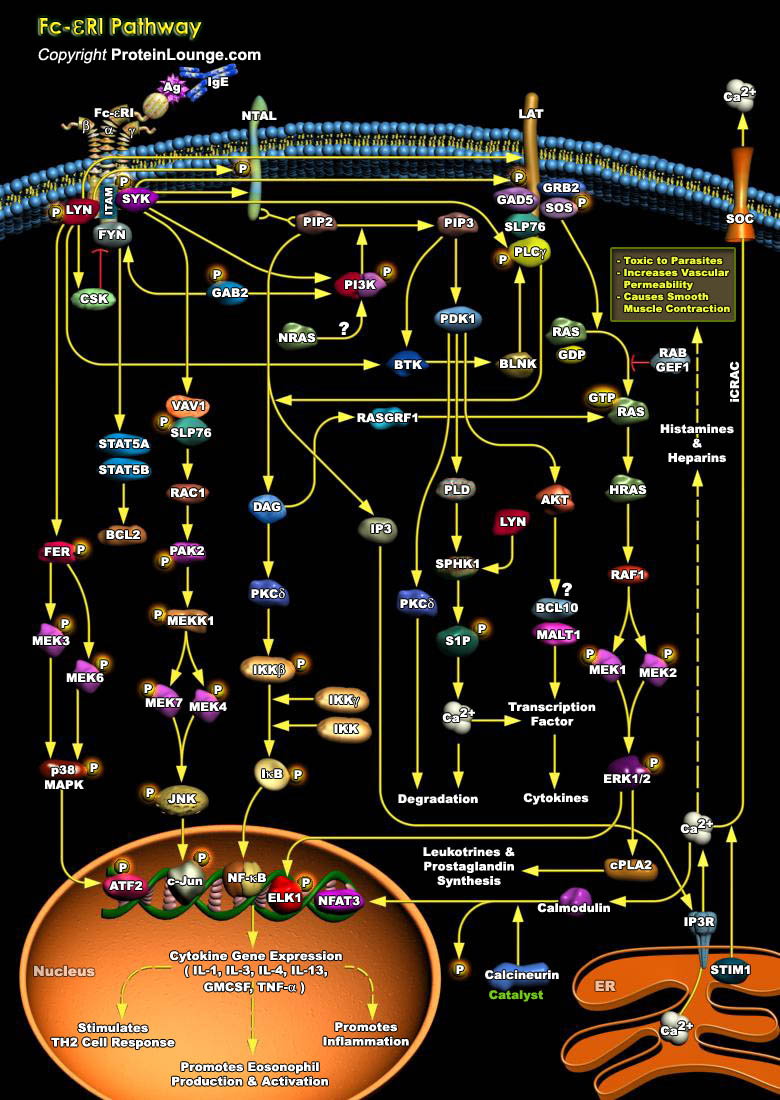
FcεRI is constitutively expressed on mast cells as a tetrameric receptor composed of the IgE-binding α chain, the membrane-tetraspanning β chain, and the disulfide-linked homodimer of the γ chains. The level of expression of the FcεRI on the mast cell surface can be influenced by several factors, such as IgE availability or IgE binding. Antigen (Ag) ligation of IgE-bound FcεRI initiates phosphorylation cascades that cause profound morphological and transcriptional modifications. As FcεRI lacks intrinsic tyrosine kinase activity, its activation requires the downstream phosphorylation of several Src kinases (LYN, SYK, and FYN) (Ref.1).Allergic rhinitis, asthma, atopic dermatitis, and food and drug allergies, which in some cases can lead[..]
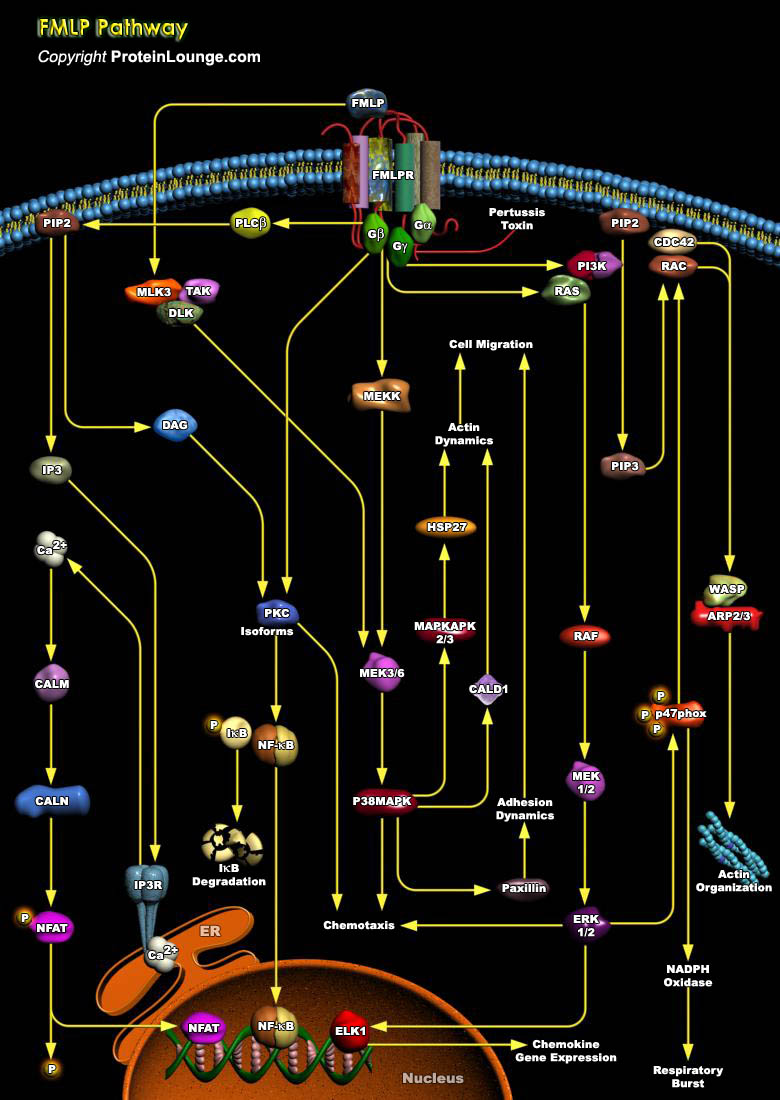
Neutrophils play an important role in the host defense by invading microbial pathogens. Upon infection neutrophils become activated through interaction with chemo attractants and cytokines. These ligands bind to a variety of cell surface receptors, including heterotrimeric GPCR (G-Protein Coupled Receptors) for fMLP (N-formyl-Met-Leu-Phe) and PAF (Platelet Activating Factor), and tyrosine kinase-associated receptors for GMCSF (Granulocyte-Macrophage Colony Stimulating Factor). Receptor activation triggers intracellular signal transduction pathways, resulting in the correct biological response, for instance, migration, phagocytosis, antibody-dependent cell mediated cytotoxicity, degranulation, superoxide production, transcriptional activation, and actin[..]
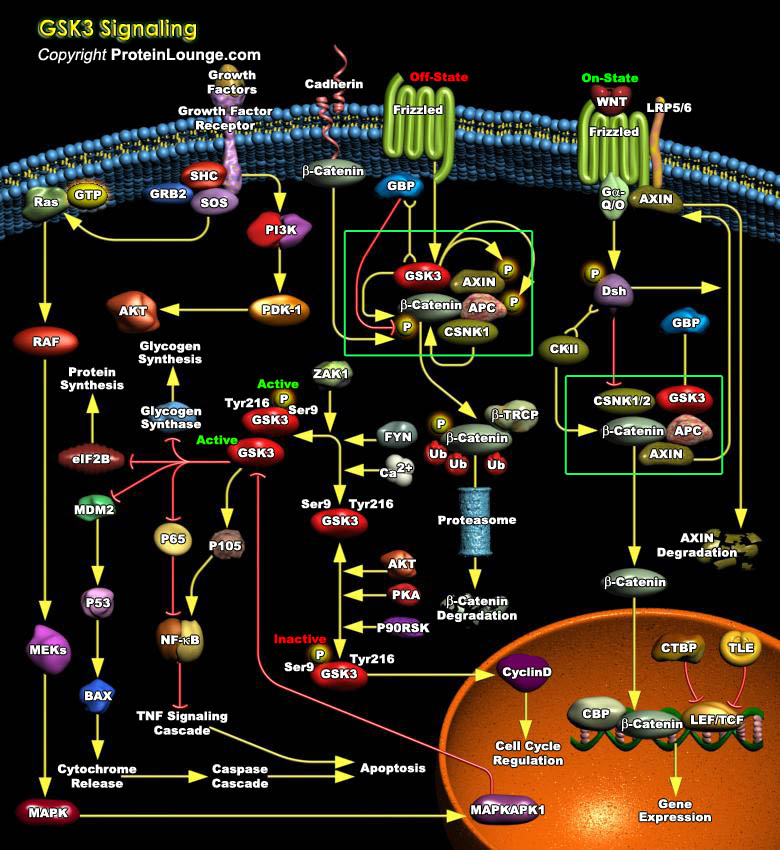
GSK3 (Glycogen Synthase Kinase-3) is a ubiquitously expressed, highly conserved serine/threonine protein kinase found in all eukaryotes. Identified originally as a regulator of glycogen metabolism, GSK3 acts as a downstream regulatory switch for numerous signaling pathways, including cellular responses to WNT, Growth Factors, Insulin, RTK (Receptor Tyrosine Kinases), Hedgehog pathways, and GPCR (G-Protein-Coupled Receptors) and is involved in a wide range of signal transduction cascades involving cellular processes, ranging from glycogen metabolism, cell development, gene transcription, protein translation to cytoskeletal organization, cell cycle regulation, proliferation and apoptosis. Unlike most protein kinases involved in signaling, GSK3 is active in unstimulated,[..]
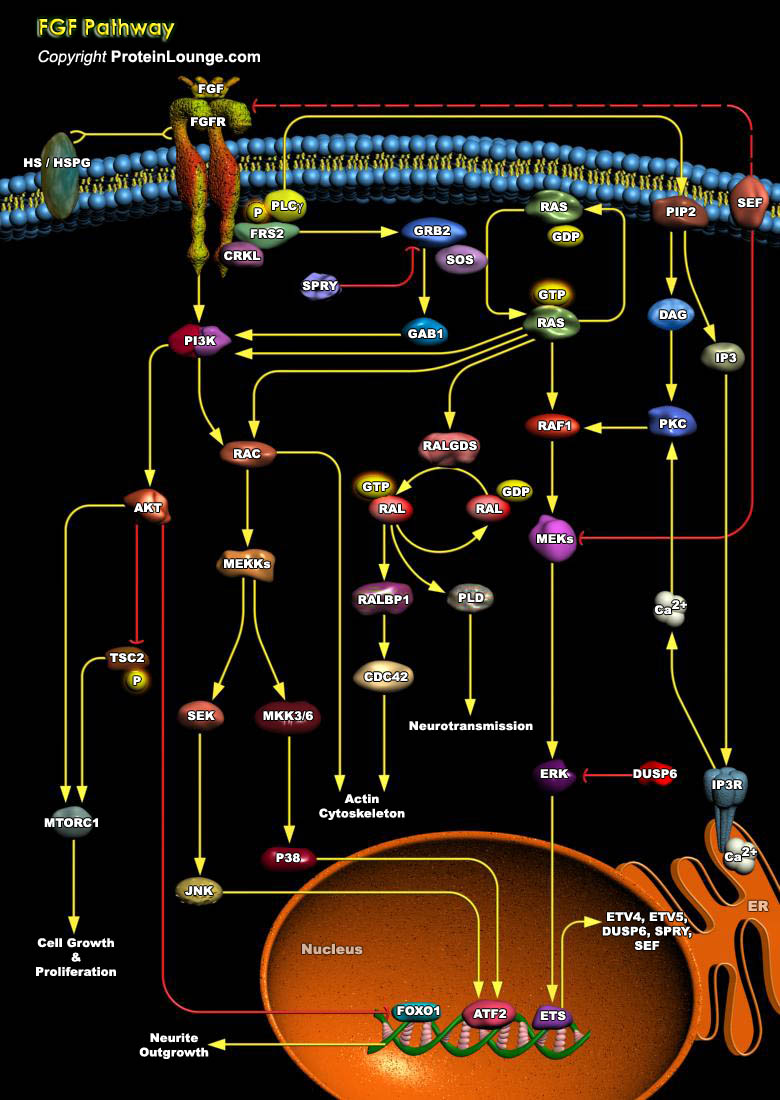
Angiogenesis, the growth of new blood vessels, plays a key role in many physiological and pathological processes, such as ovulation, embryogenesis, wound repair, inflammation, malignant tumor growth, retinopathies, rheumatoid arthritis, and angiogenesis-dependent diseases. One of the best-characterized modulators of angiogenesis is the heparin-binding FGF (Fibroblast Growth Factor) (Ref. 1).FGFs are a large family of multifunctional peptide growth factors of which there are at least 28 distinct members. The members of this peptide growth factor family have been identified in a variety of organisms and play pivotal roles in many cellular processes including mitogenesis, differentiation, migration, and cell survival During embryonic development, FGFs play a critical role[..]
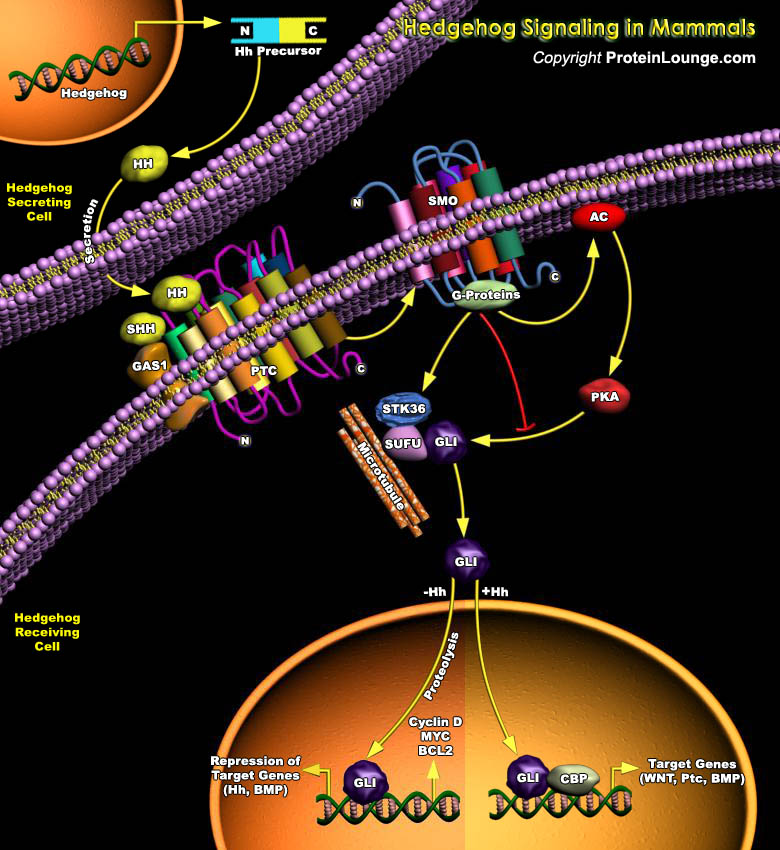
Controlled cell proliferation is a predominant theme in normal embryonic and post-embryonic development, and, in many instances, cell-type specification and cell proliferation are intimately coupled. Several secreted intercellular signaling proteins that behave as morphogens during pattern formation are also implicated in the regulation of the cell cycle. Hedgehogs (Hhs) are one such class of morphogens that regulate an enormous variety of developmental events in the fly and vertebrate embryo and plays a central role in several cancers.The vertebrate Hh family is represented by at least three members: Dhh (Desert Hh), Ihh (Indian Hh) and Shh (Sonic Hh), two Patched homologs, Ptc1 (Patched-1) and Ptc2 (Patched-2); and three homologs of Ci (Cubitus interruptus, a 155 kDa[..]
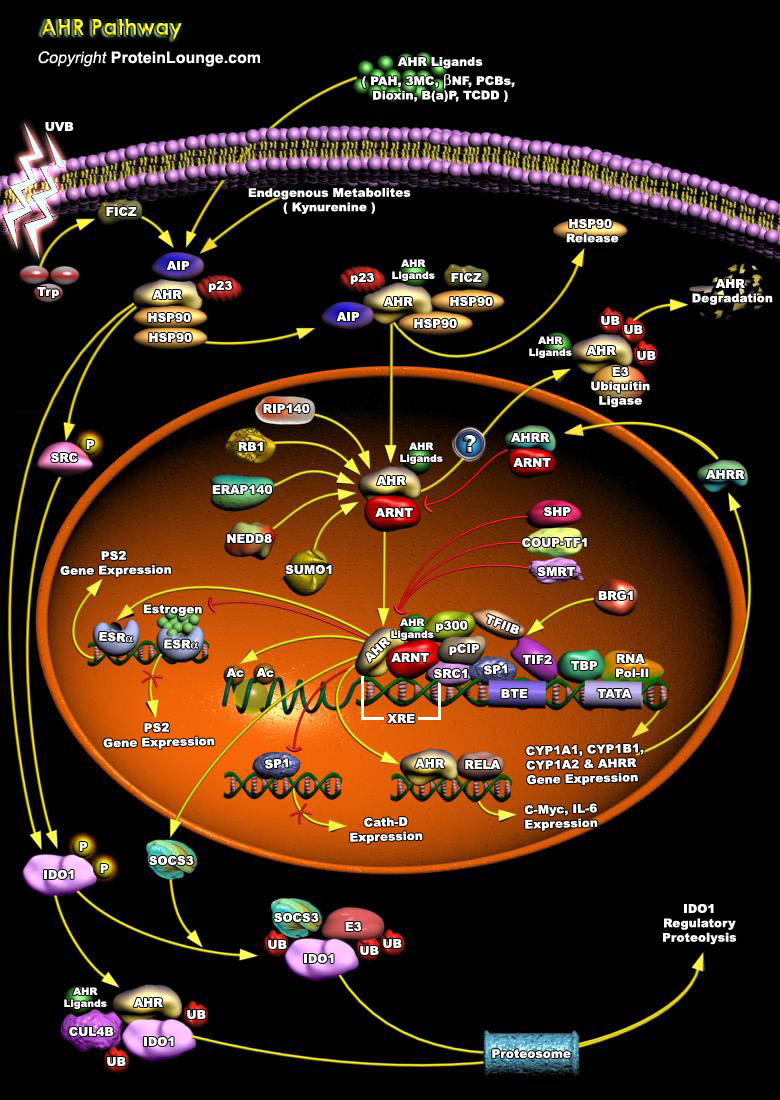
AHR is a cytosolic receptor for low molecular weight molecules, binding and becoming activated by sterically planar ligands approximately three benzene rings in size. It is maximally expressed in interface tissues including the liver, lungs, skin and gastrointestinal tract [Ref.1]. It is recognized as the culprit for most toxic responses observed after exposure to PAH (Polycyclic Aromatic Hydrocarbons), Dioxins (e.g. TCDD (2, 3, 7, 8-tetrachlorodibenzo-p-dioxin)), and PCBs(Polychlorinated Biphenyls),3MC,β-NF and B[a]P. It is also activated by FICZ, which is formed upon absorption of UVB by TRP (tryptophan) and results in dissociation of PP60 from the complex [2&3]. AHR can affect cellular signaling through interactions with various Regulatory and Signaling[..]

