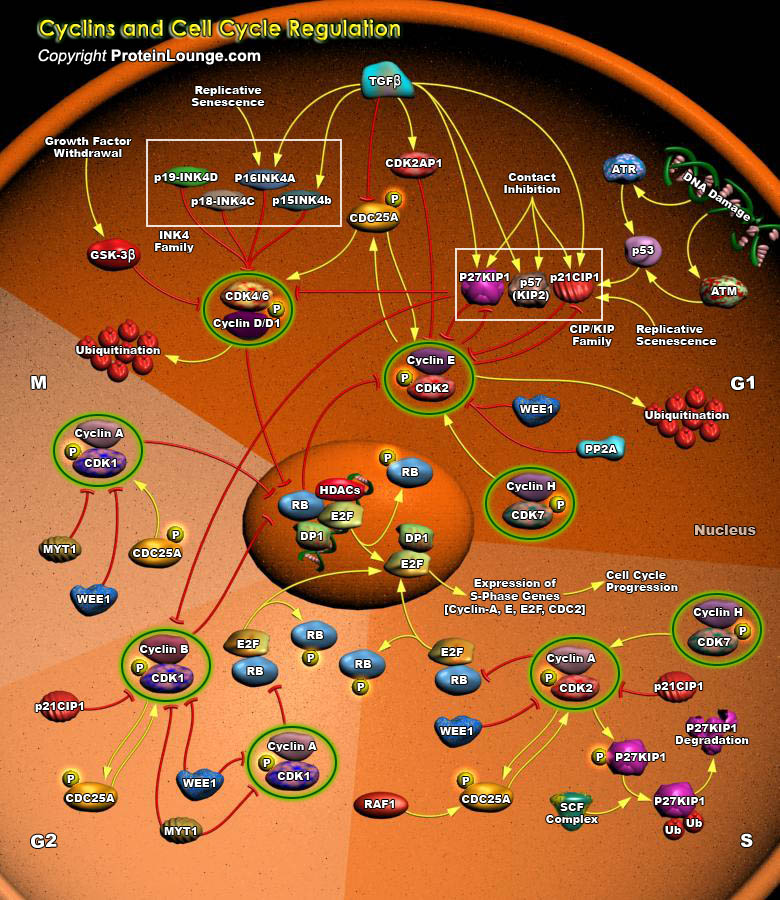
Progress in the eukaryotic cell cycle is driven by oscillations in the activities of CDKs (Cyclin-Dependent Kinases). CDK activity is controlled by periodic synthesis and degradation of positive regulatory subunits, Cyclins, as well as by fluctuations in levels of negative regulators, by CKIs (CDK Inhibitors), and by reversible phosphorylation. The mammalian cell cycle consists of four discrete phases: S-phase, in which DNA is replicated; M-phase, in which the chromosomes are separated over two new nuclei in the process of mitosis. These two phases are separated by two so called “Gap” phases, G1 and G2, in which the cell prepares for the upcoming events of S and M, respectively.The different Cyclins, specific for the G1-, S-, or M-phases of the cell cycle,[..]
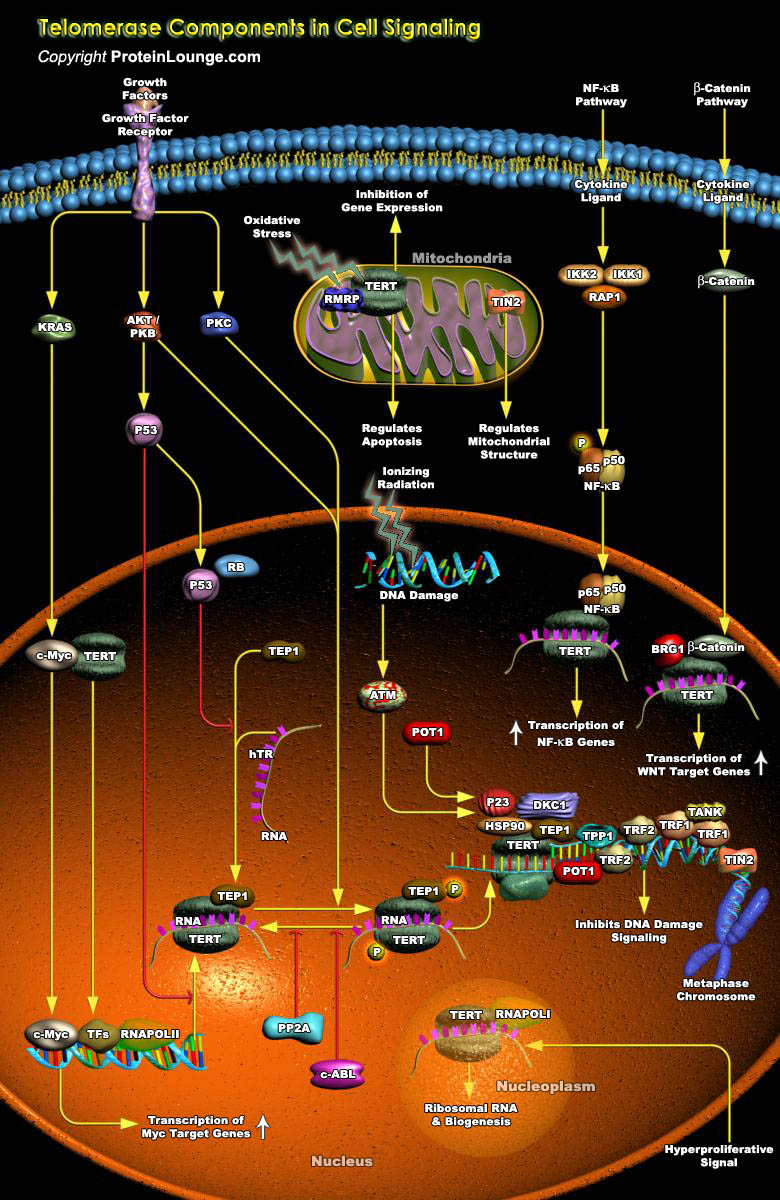
The extended growth potential of cancer cells is critically dependent upon the maintenance of functional telomeres, which are specialized chromosomal DNA-protein structures in the terminal regions of eukaryotic chromosomes (Ref.1). In order to divide, a normal cell has to replicate the entire DNA in its chromosomes. But normal cells have difficulty in copying the last few bases on the telomere. As a result, the telomere shortens with each round of DNA replication and cell division and as a cell ages, the telomere keeps shortening until it reaches a finite length. At that point cells stop dividing and this halt in growth is triggered by a gene p53 that is activated in response to DNA damage. A telomere that becomes too short no longer protects the chromosome from DNA[..]
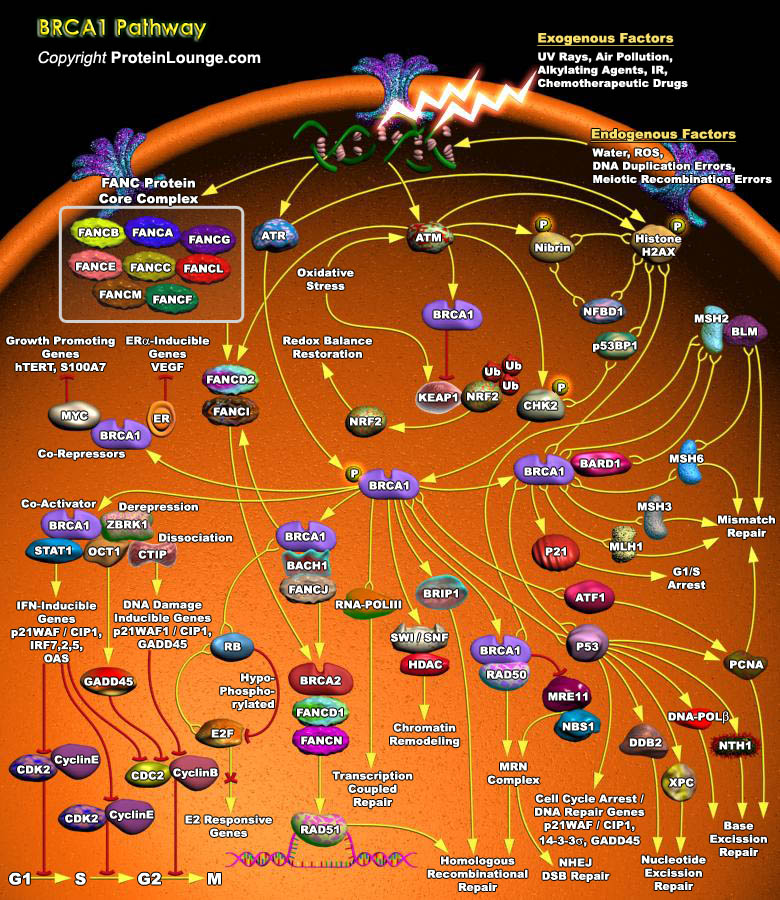
BRCA1(Breast Cancer Susceptibility Protein-1) is a versatile protein that links DNA damage sensing and DDR effectors. BRCA1 interacts with tumour suppressors, DNA repair proteins and cell cycle regulators through its various functional domains and thereby has diverse roles in multiple DNA repair pathways (particularly HR, NHEJ and SSA (single-strand annealing)) and in checkpoint regulation. BRCA1 contains an amino-terminal RING domain that has E3 ubiquitin ligase activity (which catalyses protein ubiquitylation) and a BRCT(BRCA1 C-Terminal) domain that facilitates phospho-protein binding.BASC (BRCA1-Associated Genome Surveillance Complex), a super complex of BRCA1, is key to recognizing and repairing DNA damage. This complex includes tumor suppressors and DNA damage[..]
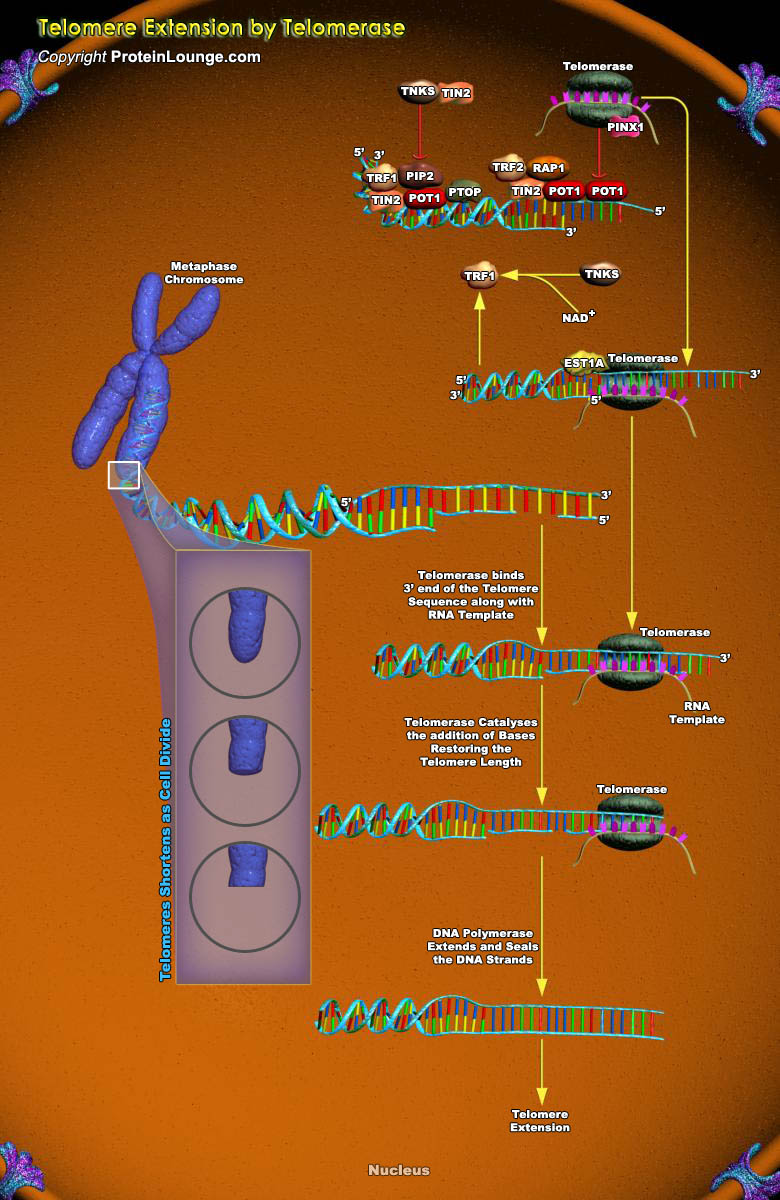
Unlimited replicative potential and widespread genomic disarray are among the most common characteristics exhibited by human cancer cells. Although several distinct molecular pathways regulate specific aspects of each of these phenotypes, specialized chromosomal terminal structures, termed telomeres act as essential regulators of both cell life span and chromosomal integrity (Ref.1).Telomeres are dynamic DNA-protein complexes that cap the ends of linear chromosomes, preventing detrimental chromosome rearrangements and defending against genomic instability and the associated risk of cancer. Telomeres shorten every time a cell divides because of incomplete DNA replication and DNA end processing. When telomere length reaches a critical point, cells stop dividing and[..]
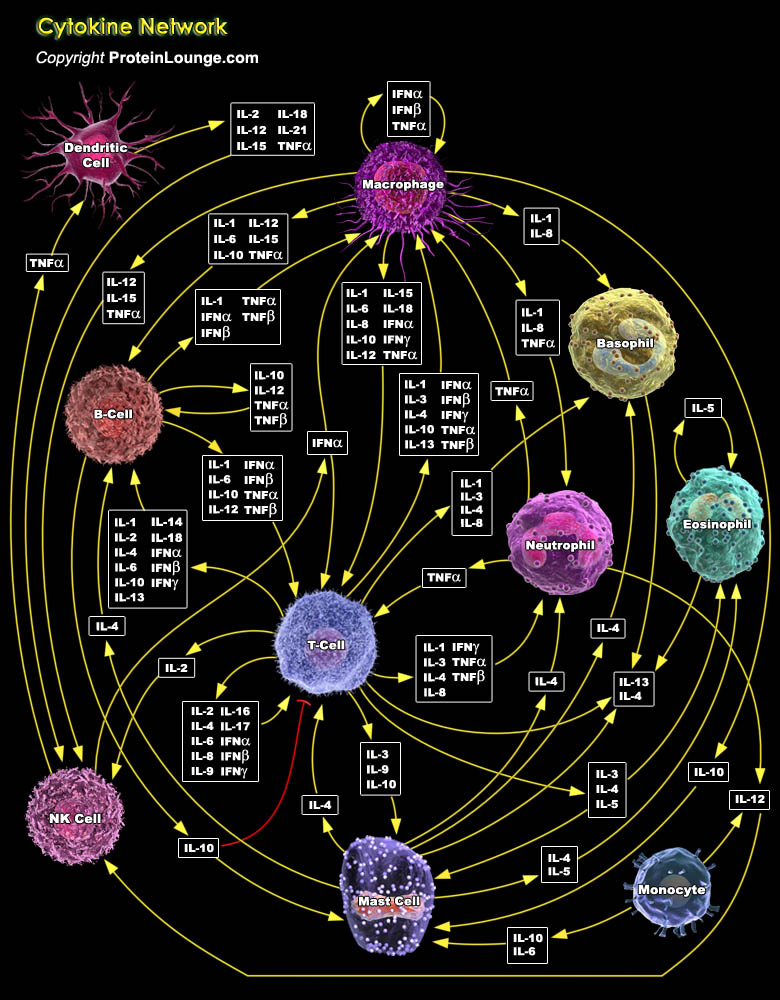
The immune system recognizes the presence of pathogens by several proteins that bind to molecules secreted by the pathogen or carried on their surface. The cells responsible for these immune responses include the B-Cells, T-Cells, macrophages, neutrophils, basophils, eosinophils, endothelial cells, or mast cells (Ref.1). These cells have distinct roles in the immune system, and communicate with other immune cells by cytokines, which control proliferation, differentiation and function of cells of the immune system. Cytokines provide cells with the ability to communicate with one another and orchestrate complex multicellular behaviour. Cytokines play an important role in normal homeostatic tissue function and dysregulation of these cytokine networks is associated with[..]
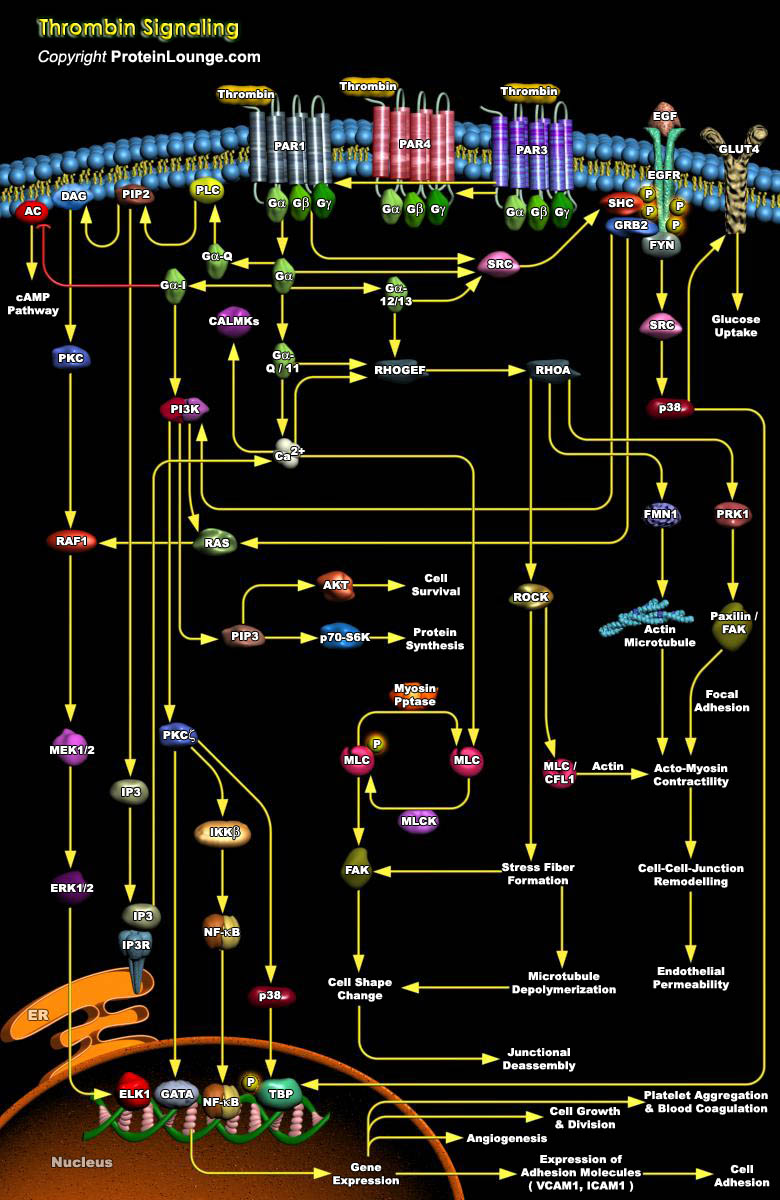
Thrombin is a multifunctional serine protease involved in a number of pathophysiological processes that include blood clotting, inflammation, repair processes and tumor metastasis. In brain, thrombin regulates the viability of neurons and astrocytes by increasing survival under conditions of hypoglycemia and oxidative stress and inducing apoptosis under other conditions. Thrombin is also chemotactic for macrophages and mitogenic for smooth muscle cells, fibroblasts, and astrocytes and induces secretion of growth factors and cytokines from fibroblasts and smooth muscle cells. Most of the thrombin-mediated effects are preceded by morphological changes in cells that follow activation of PARs (Protease-Activated Receptors) (Ref.1).PARs are a unique class of heterotrimeric,[..]
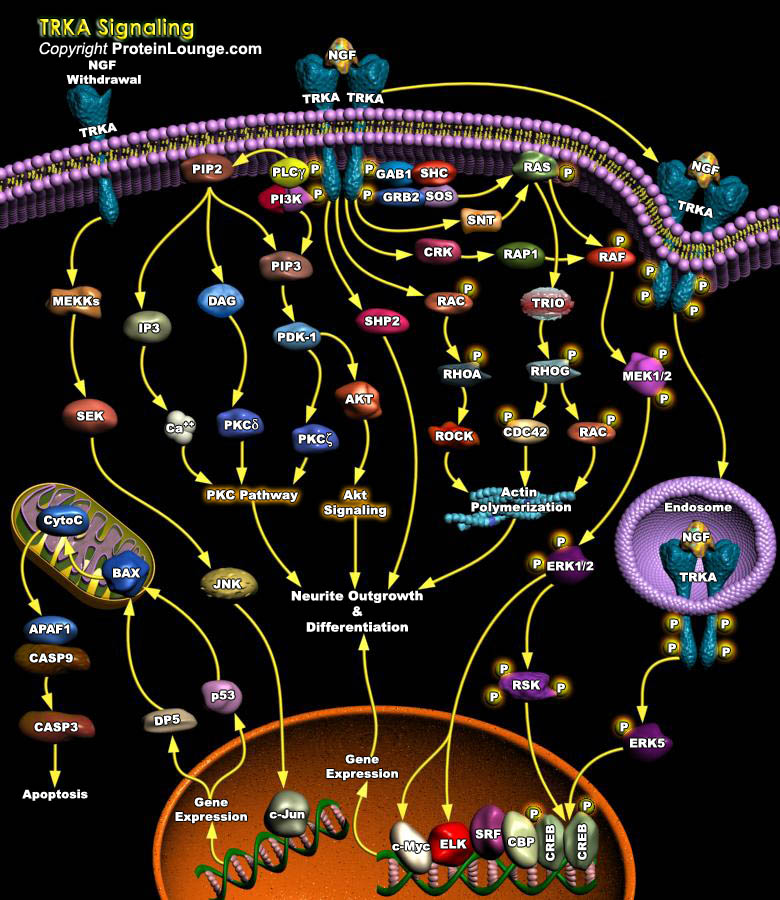
A recurring theme in neurobiology is the role of a set of molecules that support proliferation, differentiation and survival of neurons. These molecules, collectively referred to as neurotrophins, are essential for the development and maintenance of the vertebrate nervous system, mediating their signal into the cell by specific interaction with tyrosine kinase receptors of the TRK (Tyrosine Kinase Receptor) family. TRK family is composed of related transmembrane tyrosine receptor kinases that specifically bind neurotrophins: TRKA binds NGF (Nerve Growth Factor); TRKB binds BDNF (Brain Derived Neurotrophic Factor), NT3 (Neurotrophin-3) and NT4/5 (Neurotrophin-4/5); and TRKC binds NT3. Out of these, TRKA forms a high-affinity binding site for NGF, the best characterized[..]
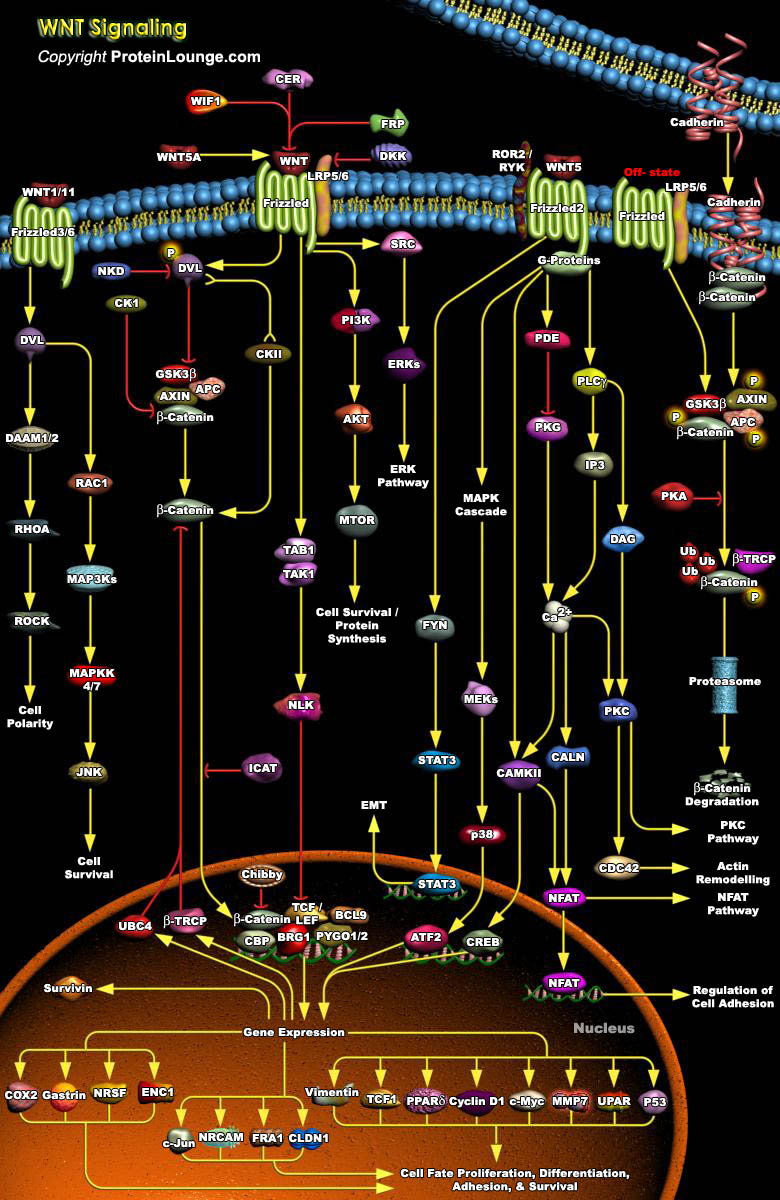
WNT signaling pathways play essential roles in cellular proliferation, differentiation and cell migration during embryonic development. The importance of WNT signaling is indicated by conservation of its molecular components across organisms ranging from nematodes to humans. WNT pathways are classified into canonical WNT/CTNNB or non-canonical (β-catenin-independent) pathways. Canonical WNT/β- catenin signaling is the most studied, and is mediated by nuclear translocation of its central effector CTNNB and Non-canonical WNT signaling occurs independently of CTNNB–TCF/LEF and is stimulated by WNT ligands that bind to a receptor complex of FZD, ROR1/2 or RYK.WNT pathway is implicated in a variety of cancers (Ref.1 and 2).WNT ligands signal via seven[..]
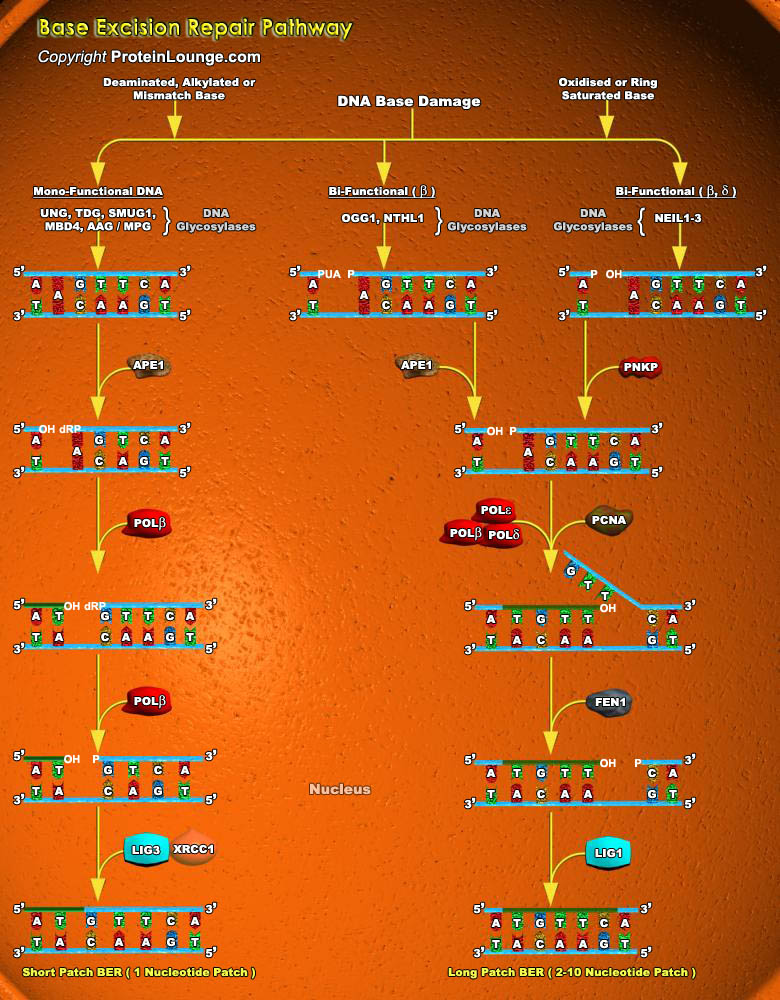
Cells are constantly under threat from the cytotoxic and mutagenic effects of DNA damaging agents that result from either endogenous sources (cellular metabolic processes) or exogenous sources (environmental factors). Endogenous sources of DNA damage include hydrolysis, oxidation, alkylation, and mismatch of DNA bases; sources for exogenous DNA damage include ionizing radiation (IR), ultraviolet (UV) radiation, and various chemicals agents (Ref.1). Repairing damage in DNA from anything that causes a mutation, such as UV radiation and tobacco smoke, is a fundamental process that protects our cells from becoming cancerous. The major forms of DNA damage include SSB (Single-strand Breaks), DSB (Double-strand Breaks), alteration of bases, hydrolytic depurination, hydrolytic[..]
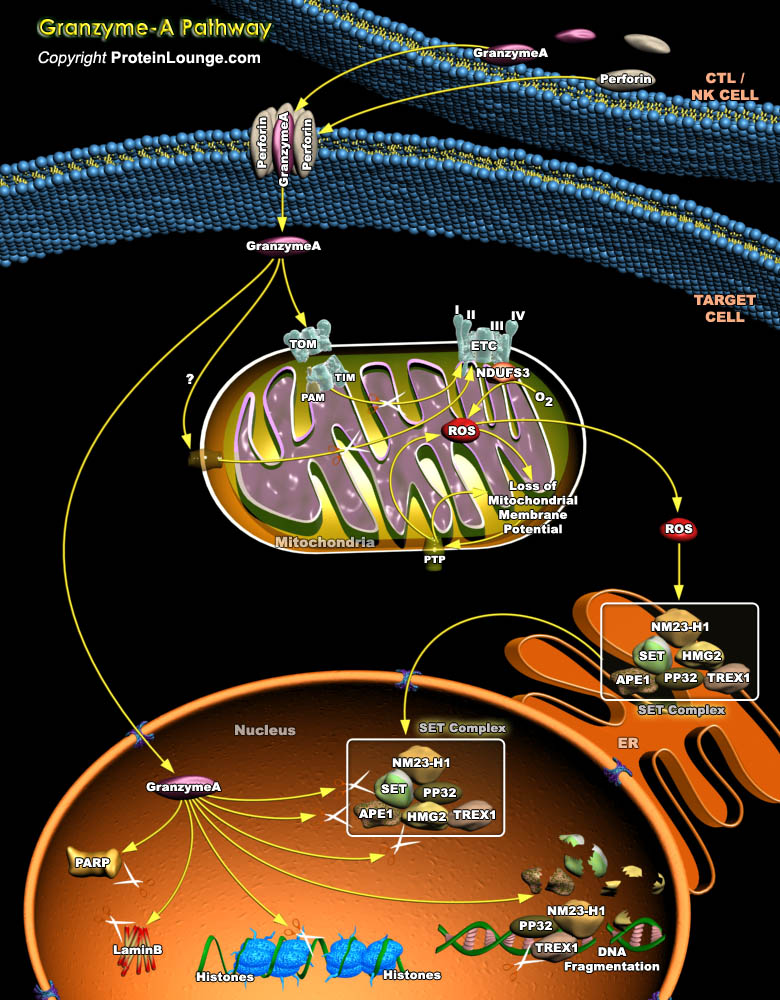
CTLs (Cytotoxic T Lymphocytes) and NK (Natural Killer) cells are the key immune effectors that eradicate infected cells or tumors. To destroy these targets, CTLs and NK cells mostly use the granule exocytosis pathway, which releases perforin and Granzymes from cytolytic granules into the immunological synapse formed with the target. Granzyme-A and Granzyme-B, the most abundant Granzymes, are delivered to the target cell cytosol through perforin and independently induce cell death. The tryptase Granzyme-A activates cell death through a caspase-independent mechanism. Granzyme-A causes characteristic features of apoptosis, including membrane blebbing, loss of mitochondrial transmembrane potential, nuclear fragmentation and chromatin condensation; however, instead of[..]
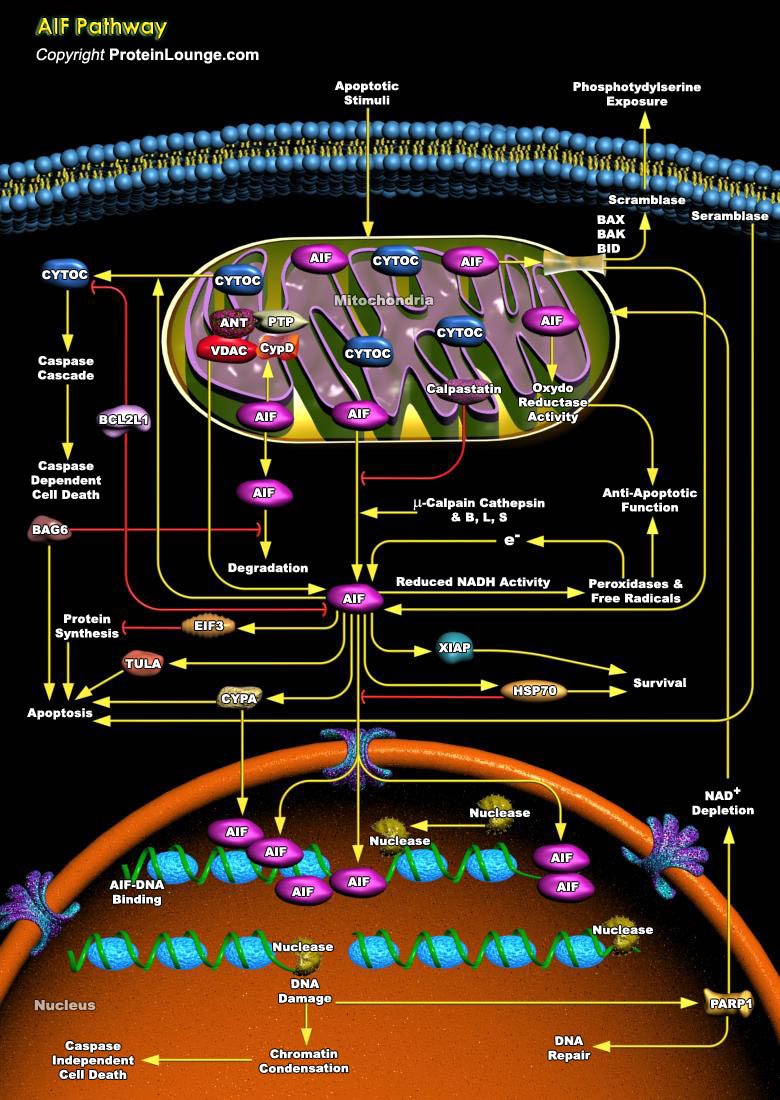
AIF (apoptosis-inducing factor), is a phylogenetically old flavoprotein which is confined to the mitochondrial intermembrane space in healthy cells but upon lethal signaling, it translocates, via the cytosol, to the nucleus where it binds to DNA and provokes Caspase-independent chromatin condensation [Ref.1]. Proteolysis of the membrane tether in mature AIF can be mediated by local or cytoplasmic proteases entering the intermembrane space upon permeabilization of the outer membrane. Mitochondrial membrane permeabilization leads to the release of other IMS (Intermembrane Space) proteins, such as CYCS (Cytochrome-C), SMAC (Second Mitochondria-Derived Activator of Caspase12)/DIABLO and ENDOG that take part in the apoptotic process. BCL2L1 (Bcl2 Related Protein Long[..]
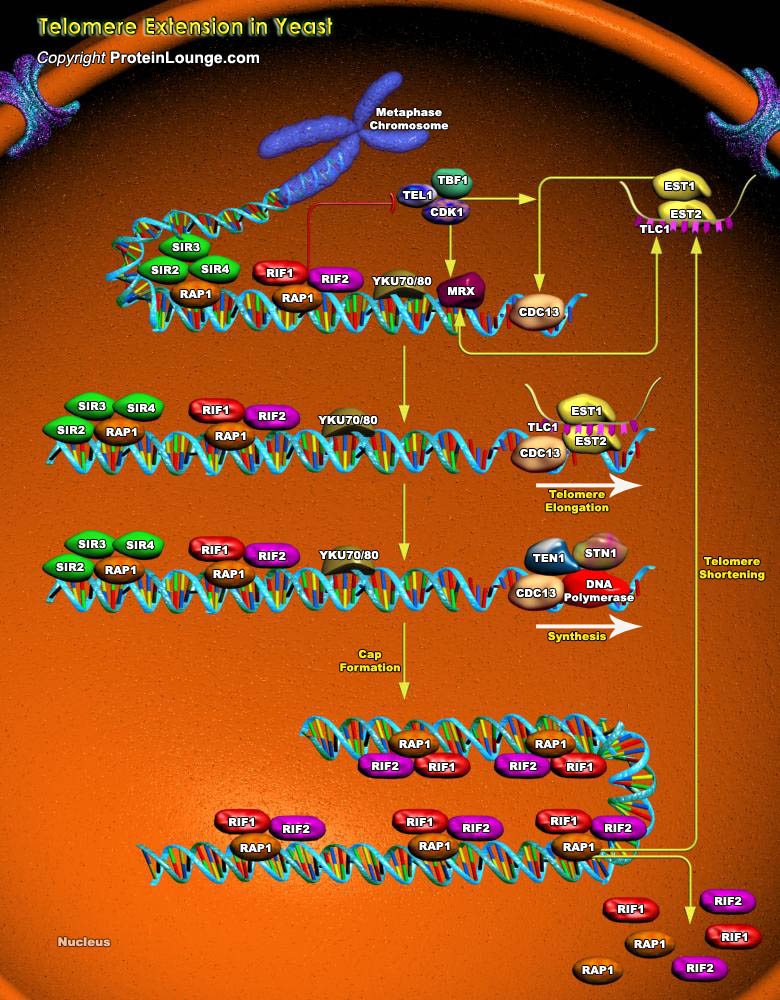
Telomeres are specialized nucleoprotein structures found at the ends of linear eukaryotic chromosomes. Telomeres confer stability to chromosomes by preventing nucleolytic degradation and recombination. They also function in chromosomal localization, nuclear architecture, and repression of nearby genes. The telomeric DNA of most organisms consists of simple tandem repeats that are rich in dG and dT residues on the 3' end-containing strand. This strand is synthesized by a ribonucleoprotein complex called telomerase, an enzyme that is critical to the maintenance of telomere length and function (Ref.1).The telomeres of the budding yeast Saccharomyces cerevisiae contain ~300 base pairs of double-stranded (TG1-3/C1-3A) sequences. Telomerase recruitment is mediated by a[..]

