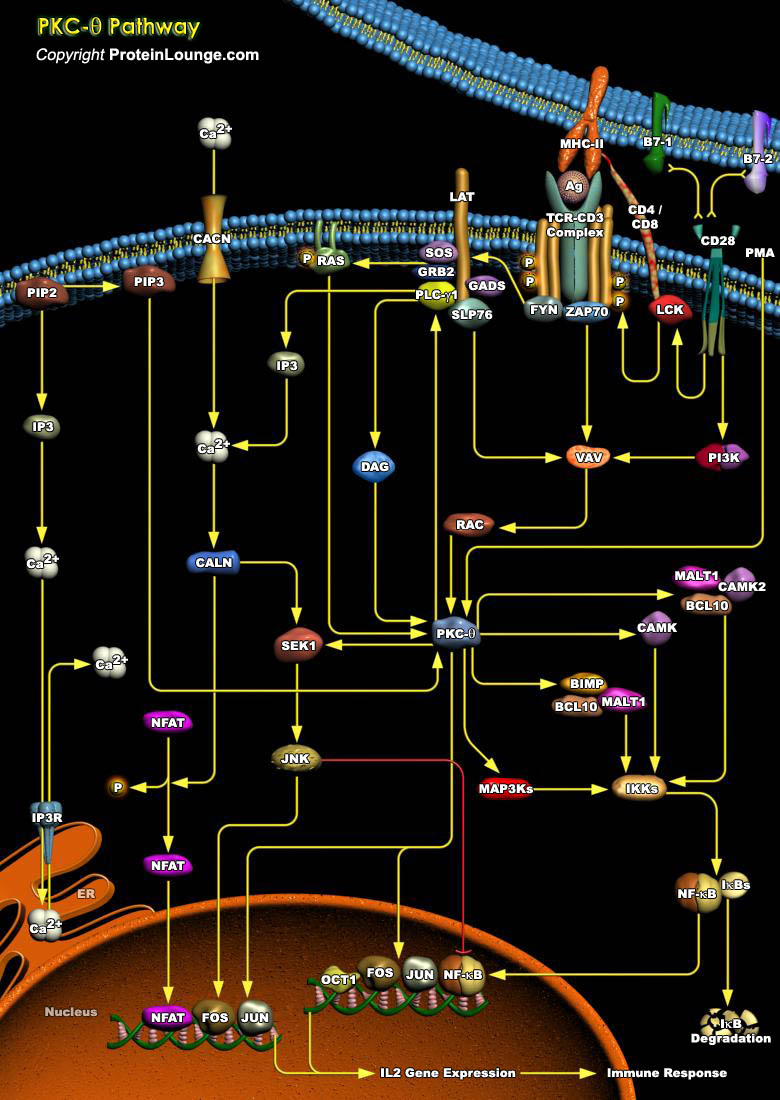
An effective immune response depends on the ability of specialized immunocytes to identify foreign molecules and respond by differentiation into mature effector cells. A cell-surface antigen recognition apparatus and a complex intracellular receptor-coupled signal-transducing machinery mediate this tightly regulated process, which operate at high fidelity to discriminate self from nonself antigens. Activation of T-Cell requires sustained physical interaction of the TCR (T-Cell Receptor) with a MHC (Major Histocompatibility Complex)–presented peptide antigen that results in a temporal and spatial reorganization of multiple cellular elements at the T-Cell–Antigen Presenting Cell contact region, a specialized region referred to as the IS (Immunological[..]
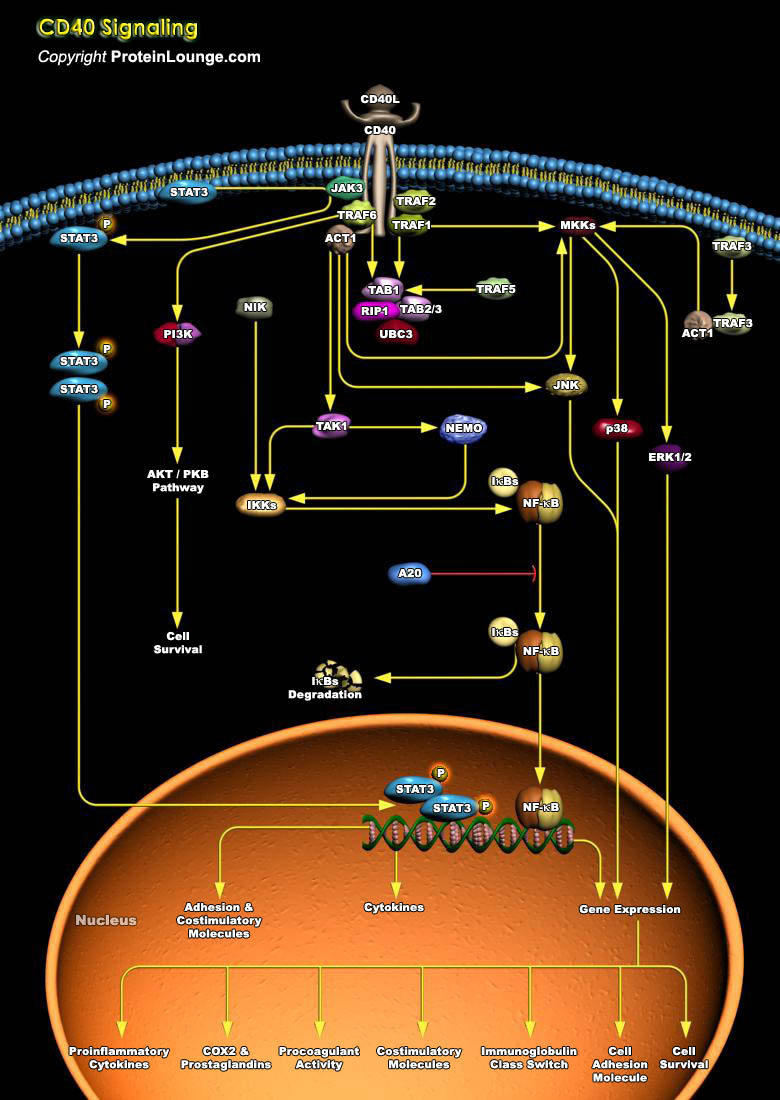
CD40, a TNFR (Tumor Necrosis Factor Receptor) family member, conveys signals regulating diverse cellular responses, ranging from proliferation and differentiation to growth suppression and cell death. First identified and functionally characterized on B-Cells, CD40 is expressed on a plethora of different cell types, including B-Cells, macrophages, dendritic cells, endothelial cells, and fibroblasts, and this widespread expression accounts for the central role of CD40 in the regulation of immune response and host defense (Ref.1). Binding of CD40 with its counter receptor, CD154 (also termed CD40L [CD40 ligand] or GP39), acts on Antigen presenting cells and T-Cells in a bi-directional fashion, mediating both humoral and cellular immune responses (Ref.2). Unique to[..]
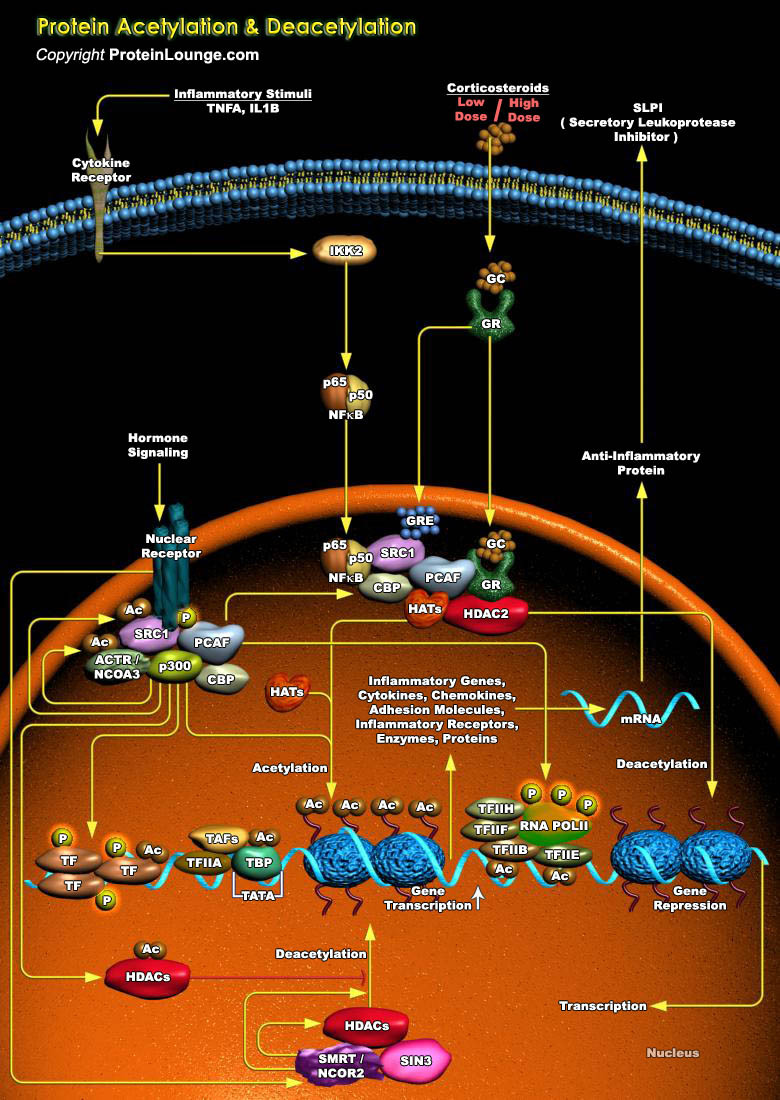
Eukaryotic transcription is a highly regulated process, and acetylation plays a major role in this regulation. Acetylation can occur on histones, DNA-binding TF (Transcription Factors), acetylases, nuclear import factors, non-nuclear proteins (Alpha-tubulin) and proteins that shuttle from the nucleus to the cytoplasm, such as the Importin-Alpha family of nuclear import factors. Acetylation can modify the recognition of DNA, the stability of proteins and the interaction between proteins. They regulate different cellular processes, such as microtubule function or nuclear import (Ref.1). By modifying chromatin proteins and transcription-related factors, these acetylases regulate diverse functions, including DNA recognition, protein-protein interaction, protein stability,[..]

The DNA damage response network involves several kinases to inactivate CDK/ cyclin complexes and result in cell cycle arrest for DNA repair. In the presence of DNA damage or incomplete DNA replication, eukaryotic cells activate cell cycle checkpoints that temporarily halt the cell cycle to permit DNA repair or completion of DNA replication to take place. In the presence of extensive damage or absence of timely repair, these checkpoint-signaling pathways may also trigger a pathway that effects programmed cell death or apoptosis. DNA damage-activated cell cycle checkpoints are regulated in part by the phosphoinositide kinase family of checkpoint components, including the yeast Rad3 in Schizosaccharomyces pombe, Mec1/Tel1 in Saccharomyces cerevisiae, mammalian ATM (Ataxia[..]
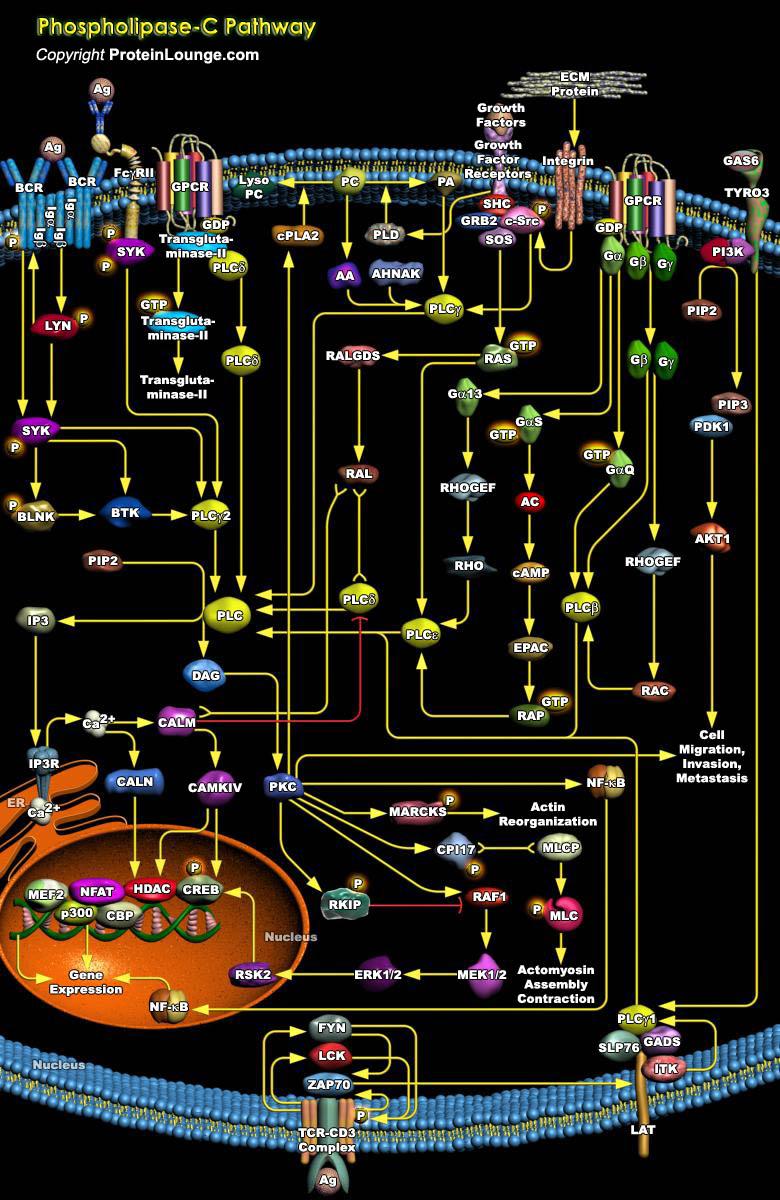
Inositol lipid-specific PLC (Phospholipase-C) isozymes are key signaling proteins in the cellular action of many hormones, neurotransmitters, growth factors, and other extracellular stimuli. PLC are soluble proteins that are partly cytosolic and partly associated with membrane. The PLC family in human is comprised of 13 subtypes. On the basis of their structure, they have been divided into six classes, PLC-Beta (Beta 1, 2, 3 and 4), PLC-Gamma (Gamma 1 and 2), PLC-Delta (Delta 1, 3 and 4), PLC-Epsilon, PLC-Zeta and PLC-Eta (Eta1 and 2) types. The molecular weights of each are 85kDa for the Delta form, 120-155kDa for both the Beta and Gamma forms, and 230-260kDa for the Epsilon form. These groups also differ in the mechanisms by which the isozymes are activated in[..]
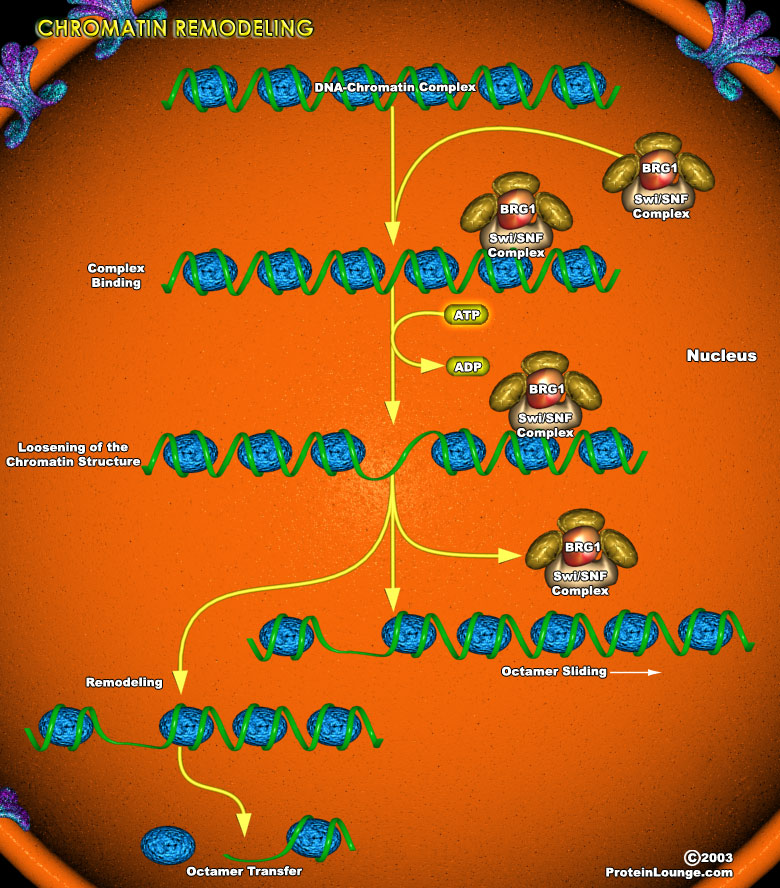
The condensation of DNA into an ordered chromatin structure allows the cell to solve the topological problems associated with storing huge molecules of chromosomal DNA within the nucleus. DNA is packaged into chromatin in orderly repeating protein-DNA complexes called nucleosomes. Each nucleosome consists of approximately 146bp of dsDNA (double-stranded DNA) wound 1.8 times around a histone octamer.Two molecules each of H2A, H2B, H3, and H4 comprise the histone ramp around which the DNA superhelix winds. Stretches of DNA upto 100bp separate adjacent nucleosomes. Multiple nuclear proteins bind to this linker region, some of which may be responsible for the ordered wrapping of strings of nucleosomes into higher-order chromatin structures (Ref.1).Chromatin assembly[..]
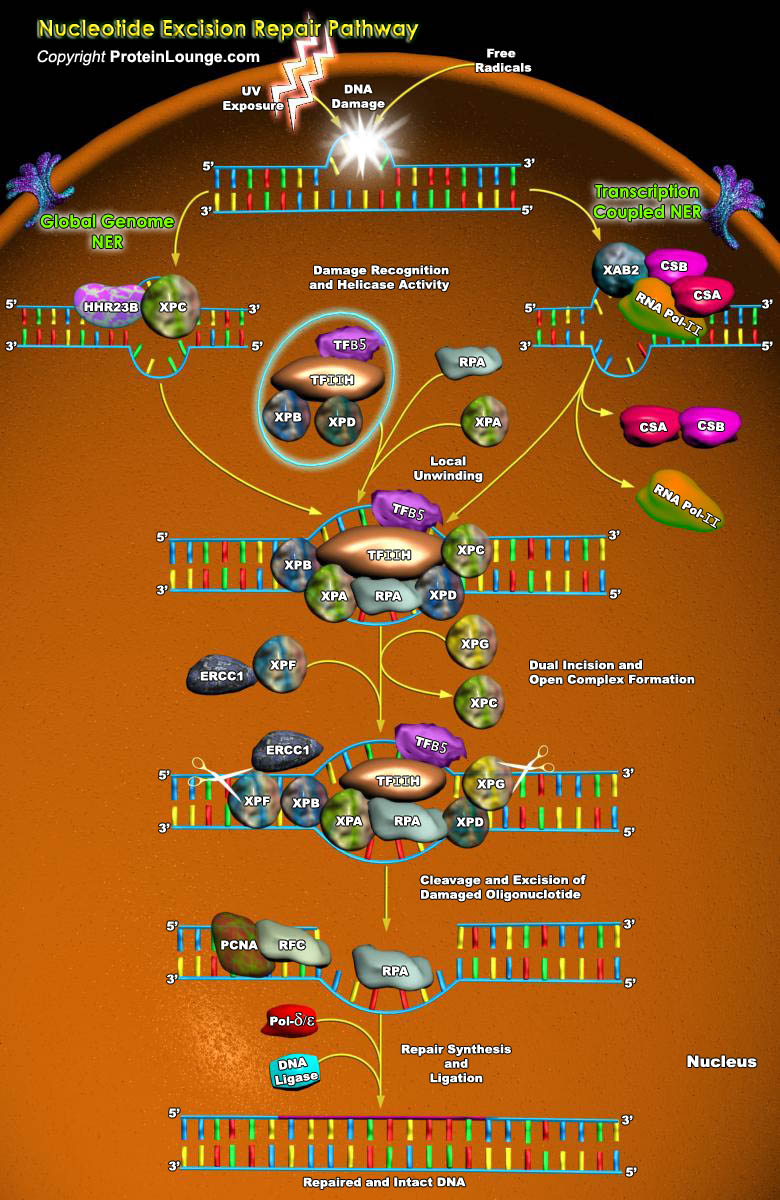
Nucleotide excision repair (NER) is the main pathway used by mammals to remove bulky DNA lesions such as those formed by UV light, environmental mutagens, and some cancer chemotherapeutic adducts from DNA. Deficiencies in NER are associated with the extremely skin cancer-prone inherited disorder xeroderma pigmentosum. Although the core NER reaction and the factors that execute it have been known for some years, recent studies have led to a much more detailed understanding of the NER mechanism, how NER operates in the context of chromatin, and how it is connected to other cellular processes such as DNA damage signaling and transcription(Ref.1). The NER process requires the action of more than 30 proteins in a stepwise manner that includes damage recognition, local[..]
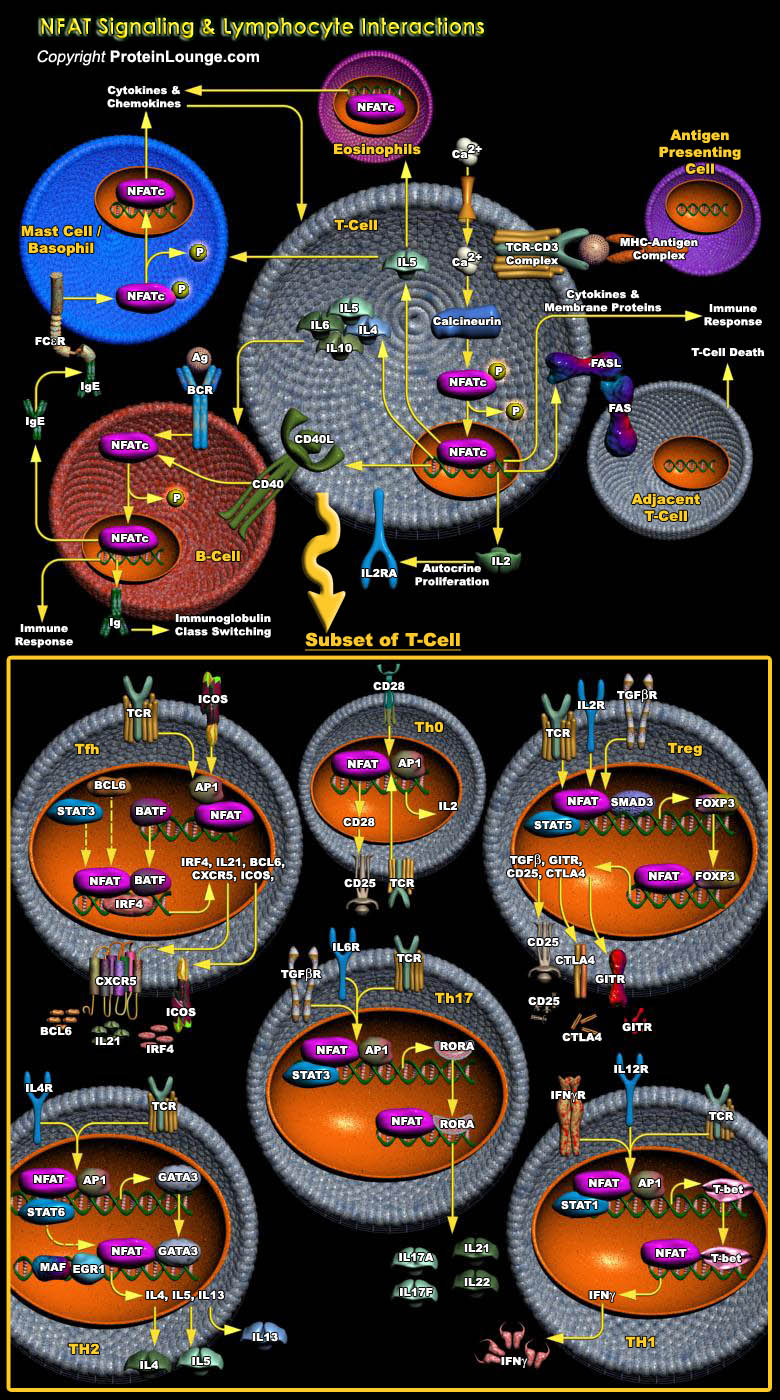
The optimum functioning of the immune system is crucial for human survival. The invading pathogens are encountered by the cells of the immune system, which include T-Cells, B-Cells, macrophages, neutrophils, basophils, eosinophils, endothelial cells, or mast cells. These cells have distinct roles in the immune system, and cell-to-cell communication among these cells is an indispensable prerequisite for the stimulation of the optimum immune response. In the process, cytokines serve as signal molecules for communication that are induced by several cell-signaling cascades. NFATs (Nuclear factors of activated T-Cells), a family of transcription factors expressed by diverse cell types of the immune system plays a pivotal role in the process. Originally described in[..]
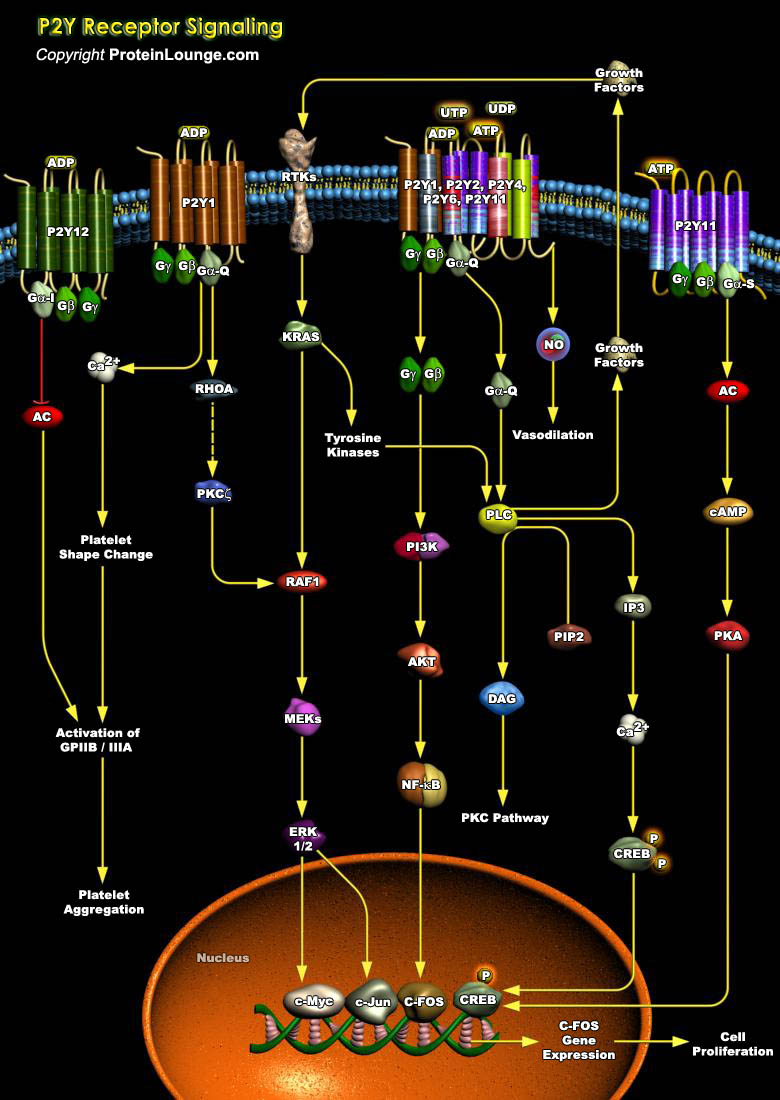
Angiogenesis plays an important role in pathological events such as tumor growth, wound healing, psoriasis, and the ischemic retinopathies that occur in diabetes and sickle cell disease. The main modulators of the angiogenesis process in adults, is achieved through signaling by peptide growth factors, however, recent researches also emphasize the contribution of purines and pyrimidines to this process. Synergistic actions of purines and pyrimidines with growth factors aid in promoting cell proliferation (Ref.1). ATP (Adenosine Triphosphate), ADP (Adenosine Diphosphate), UTP (Uridine Triphosphate), UDP (Uridine Diphosphate) and adenosine play pivotal signaling roles in these long-term events, referred to as purinergic signaling, mediated via P1 and P2 receptors.[..]
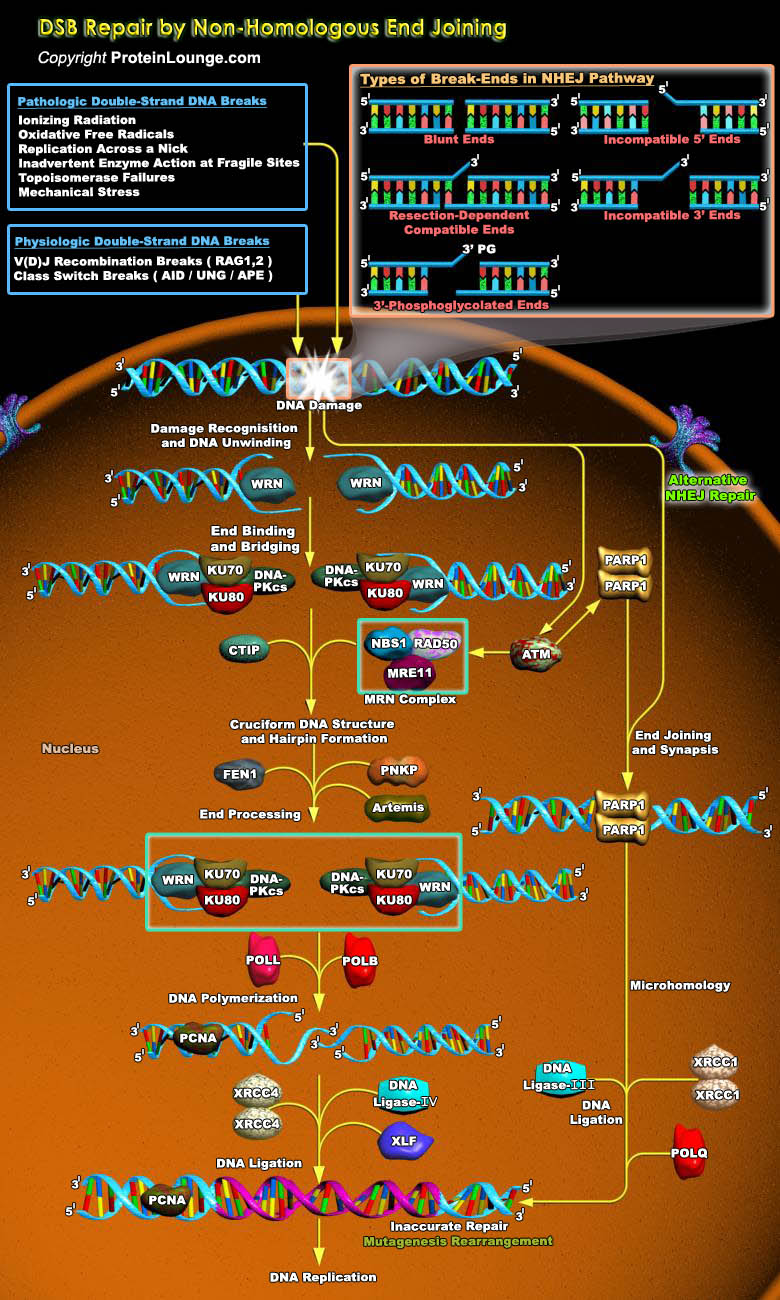
DNA double-strand breaks (DSBs) are serious lesions that threaten a loss of chromosomal content. Repair of DSBs is particularly challenging because, unlike all other lesions, the DNA substrate is inherently bimolecular. Bringing two DNA molecules together is also dangerous because local mutations and chromosome rearrangements can arise if ends are inappropriately coupled. The cell has two general strategies for repairing DSBs i.e. HR (Homologous recombination) and NHEJ (Non-homologous end joining). HR is a mechanism in which an intact homologous donor duplex is used to guide DNA synthesis across the DSB gap whereas NHEJ is defined as repair in which two DSB ends are joined by direct ligation. The resulting joints are characterized by little (less than ~10 bp) or no[..]
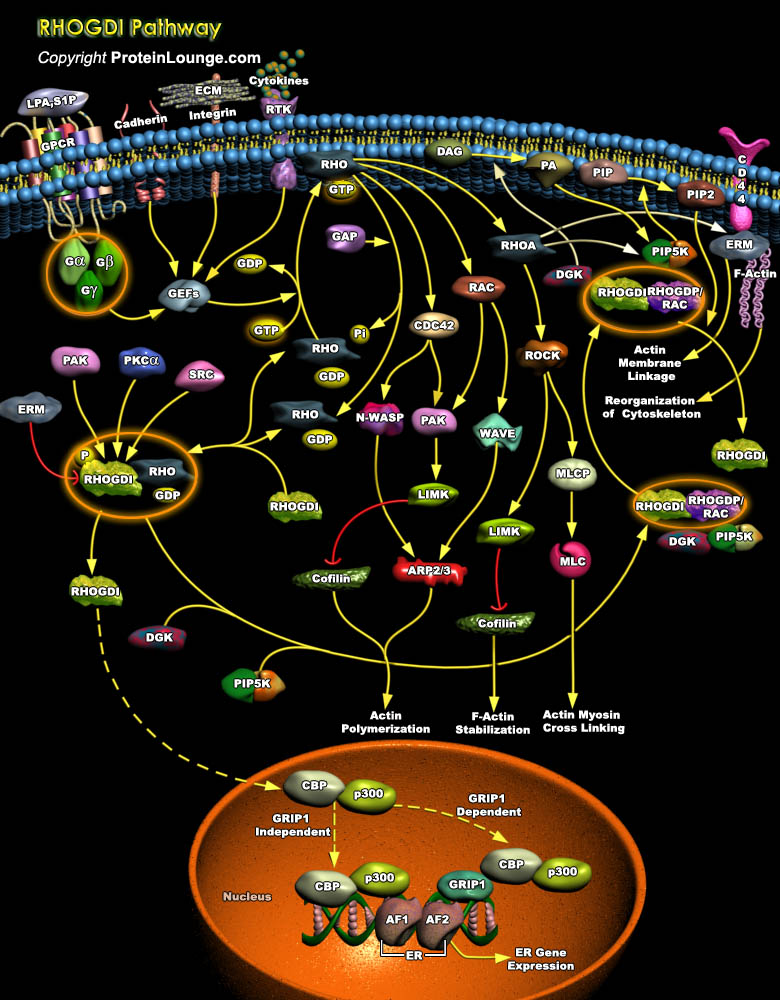
The Rho family of small GTPase proteins comprises CDC42 (Cell Division Cycle-42), Rac, and Rho. Proteins of the Rho/Rac subfamily (Rho proteins) of small GTP-binding proteins function as molecular switches that regulate a multitude of biological processes including cell proliferation, apoptosis, differentiation, migration, cytoskeletal reorganization, and membrane trafficking. Like most of the Ras-related proteins, Rho GTPases cycle between an inactive GDP-bound conformation and an active GTP- bound conformation. Cycling between the two conformations enables these proteins to act as binary switches (Ref.1). The tightly regulated GTP-binding/GTPase cycle requires the coordinated action of three regulatory proteins: 1) GEFs (Guanine Nucleotide Exchange Factors), which[..]
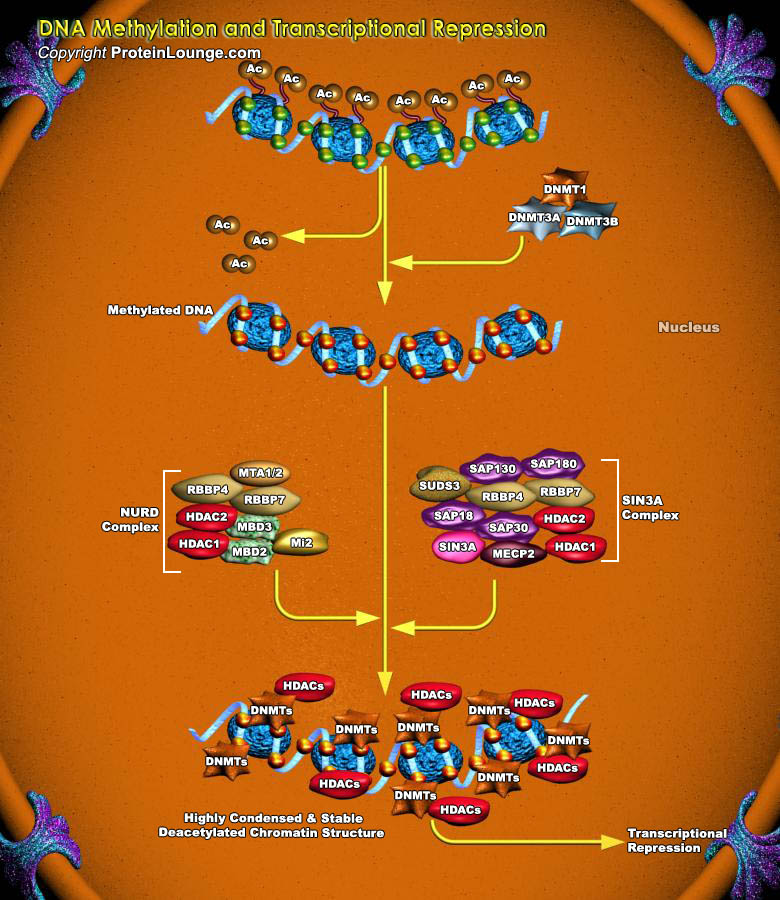
DNA methylation is one of the best characterized epigenetic modifications. In mammals it is involved in various biological processes including the silencing of transposable elements, regulation of gene expression, genomic imprinting, and X-chromosome inactivation.DNA methylation of gene promoter regions is associated with transcription repression.Transcriptional repression is an essential mechanism in the precise control of gene expression. Transcriptional repressor proteins associate with their target genes either directly through a DNA-binding domain or indirectly by interacting with other DNA-bound proteins. To inhibit transcription in a selective manner, a repressor protein can mask a transcriptional activation domain, block interaction of an activator with other[..]

