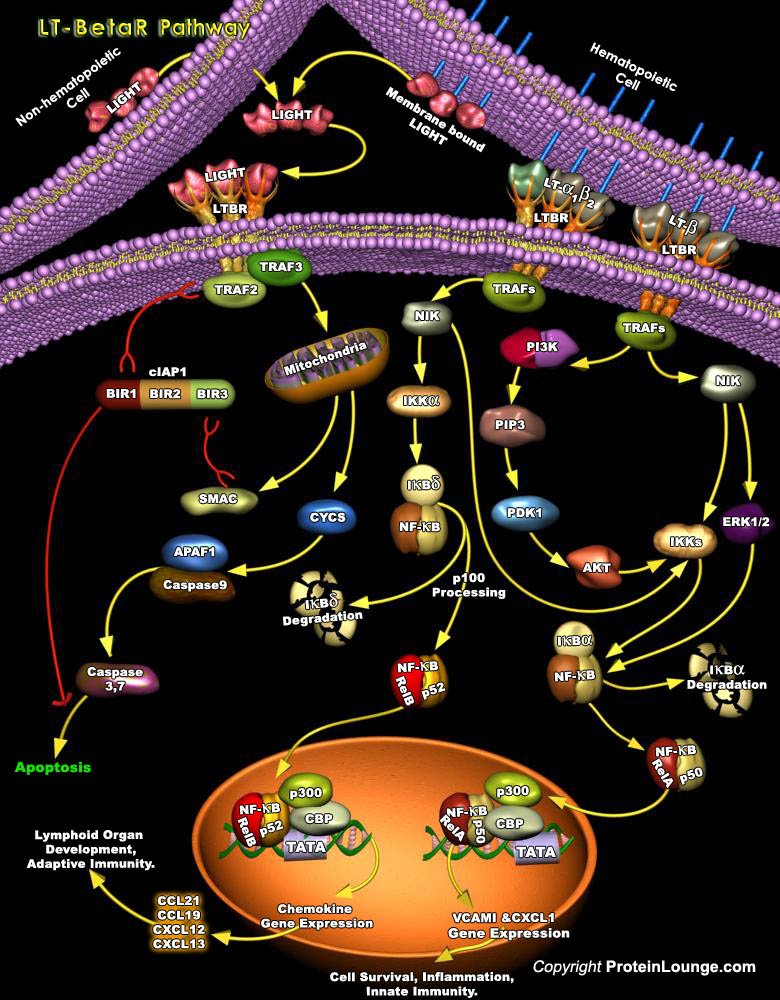
Much of the efficiency of the immune system is attributed to the high degree of spatial and temporal organization in the secondary lymphoid organs. Signaling through the LT-BetaR (Lymphotoxin-Beta Receptor) pathway is a crucial element in the maintenance of this organised microenvironment (Ref.1). LT-BetaR, a member of the TNFR (Tumor Necrosis Factor Receptor) superfamily, plays important roles in embryonic development and organization of secondary lymphoid tissues and maintenance of their architecture in adults (Ref. 2). LT-BetaR is expressed on most cell types including cells of fibroblast, epithelial, and myeloid lineages but not on T or B lymphocytes. It can bind to specific ligands, such as: the membrane form of lymphotoxin heterotrimmer, LT-Alpha1Beta2[..]
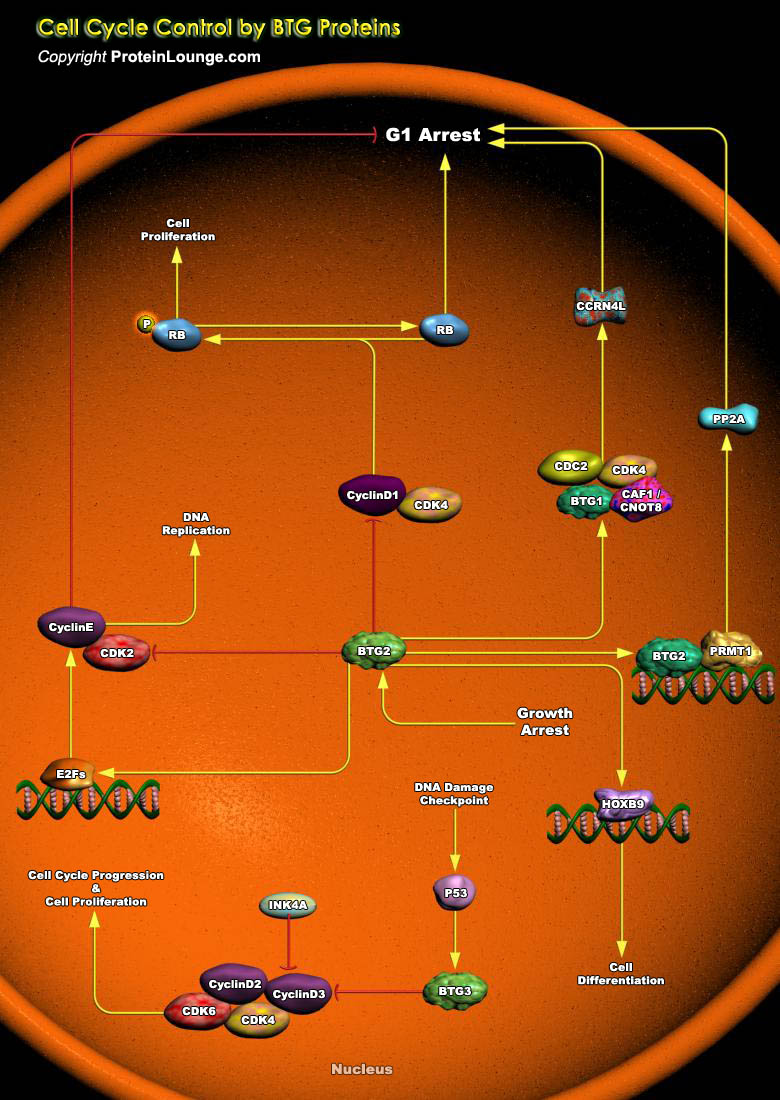
BTG2 (BTG Family Member-2) is endowed with antiproliferative activity. The expression of BTG2 in cycling cells induces accumulation of hypophosphorylated, growth-inhibitory forms of Rb (Retinoblastoma) and led to G1 arrest through impairment of DNA synthesis. Overexpression of CcnD1 (Cyclin-D1) counteracts G1 arrest. Rb is a nuclear phosphoprotein whose phosphorylation state oscillates regularly during the cell cycle. Its under-phosphorylated forms predominate in G0 and G1, while highly phosphorylated forms exist in S, G2 and M phases (Ref.1). The primary biological function of under-phosphorylated Rb is to inhibit progression toward S phase by controlling a checkpoint in late G1. In fact, under-phosphorylated Rb associates with members of the E2F family of[..]
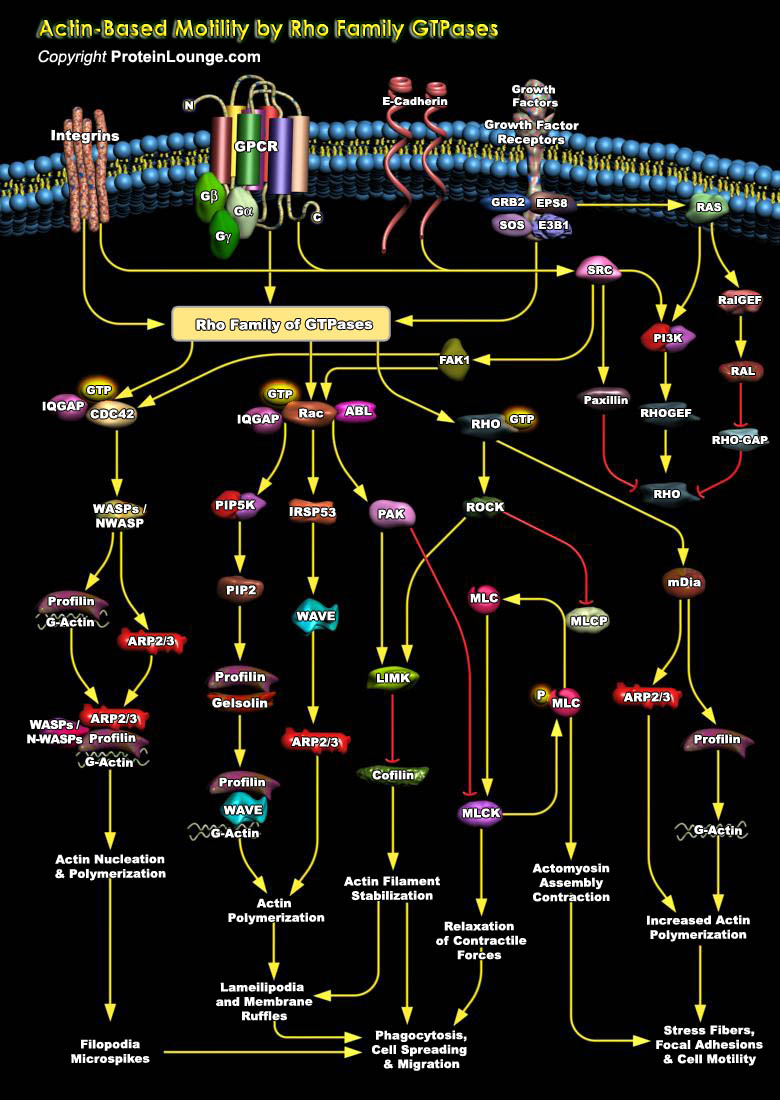
In response to a variety of extracellular stimuli, actin filament assembly at the leading edge of motile cells causes protrusion during cell crawling and chemotaxis, nerve growth and cell spreading. The actin filament network immediately under the plasma membrane in regions of rapid cellular protrusion consists of short, branched filaments while those deeper in the cortex, as well as at focal adhesions, stress fibers and in microvilli, are much longer and rarely branched (Ref.1). The dynamic organization of the actin cytoskeleton provides the force for cell motility and is regulated by small GTPases of the Rho family, in particular Rac1, RhoA and CDC42. The microtubule cytoskeleton is also polarized in a migrating cell, and in addition to organizing the actin[..]
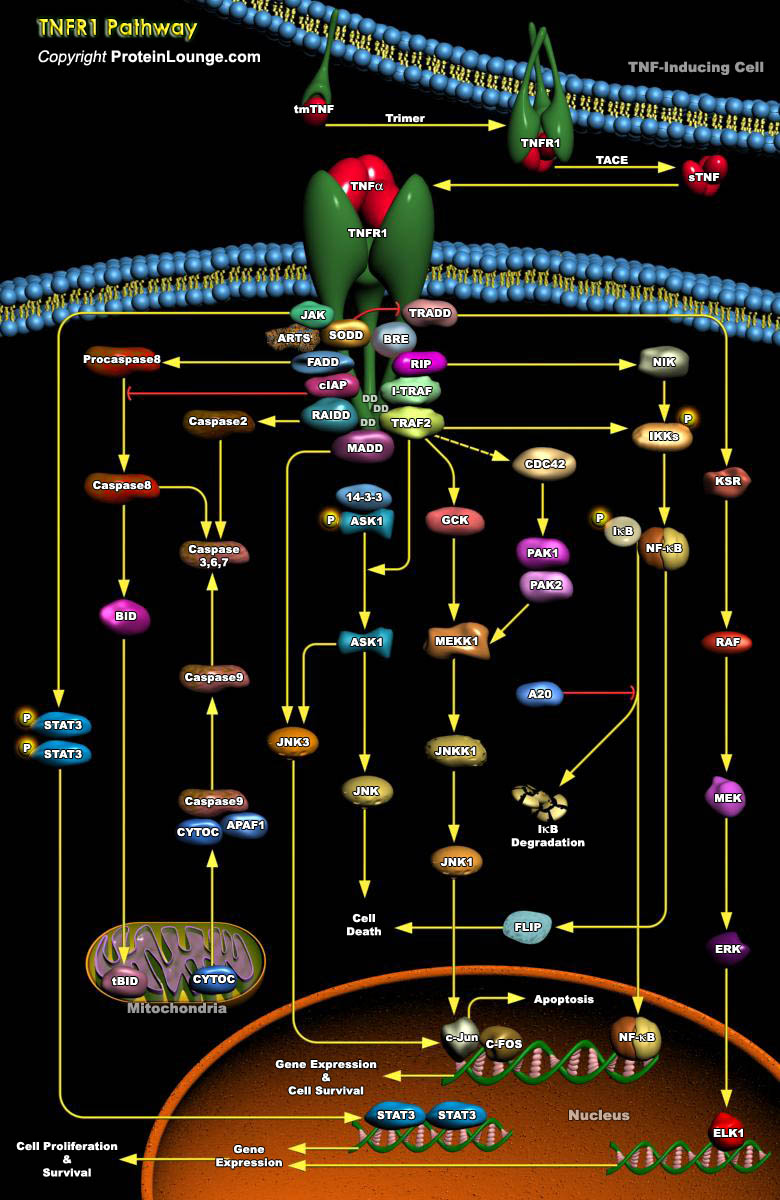
Tumor necrosis factor (TNF) is a pro-inflammatory cytokine with the capacity to induce apoptosis. It is enriched in the tumor microenvironment, promotes tumor growth and subverts innate immune responses to cancer cells. TNF is the best studied member of the TNF superfamily. TNF-alpha can bind to two related receptors, TNF receptors 1 and 2 (TNFR1 and TNFR2), which are also used by other, similar ligands. By binding to TNFR1 and TNFR2, TNF activates distinct signaling pathways important for cell proliferation, cell death and immune responses (Ref.1 and 2). TNFR1 is constitutively expressed in most cell types (Ref.3).The default effect of TNF stimulation is to activate the nuclear factor-kappaB (NF-kappaB) pathway and mediate inflammation. TNFR1 mediates the cytotoxic[..]
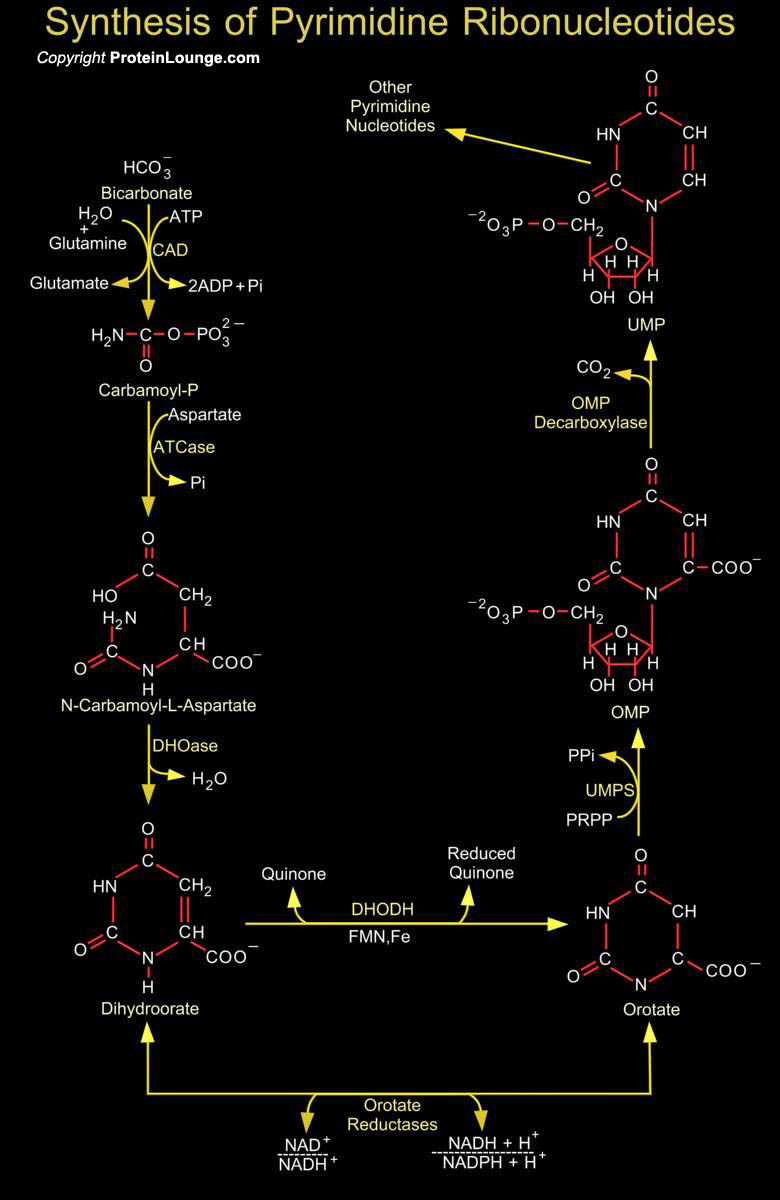
Synthesis of the Pyrimidines is less complex than that of the Purines, since the base is much simpler. Synthesis of Carbamoyl-P (Carbamoyl Phosphate) is the first reaction of Pyrimidine biosynthesis. Carbamoyl-P is formed from HCO3- (Bicarbonate) and the amide nitrogen of Glutamine by the cytosolic enzyme CPSase (Carbamoyl Phosphate Synthetase). This reaction consumes two molecules of ATP (Adenosine Monophosphate): One provides a phosphate group and the other energizes the reaction. Then condensation of Carbamoyl-P with Asp (Aspartate or L-Aspartate or Aspartic Acid) occurs to form CAA (N-Carbamoyl-L-Aspartate) which is catalyzed by ATCase (Aspartate Carbamoyltransferase). This reaction is the flux-generating step and occurs without need of ATP because Carbamoyl-P is[..]
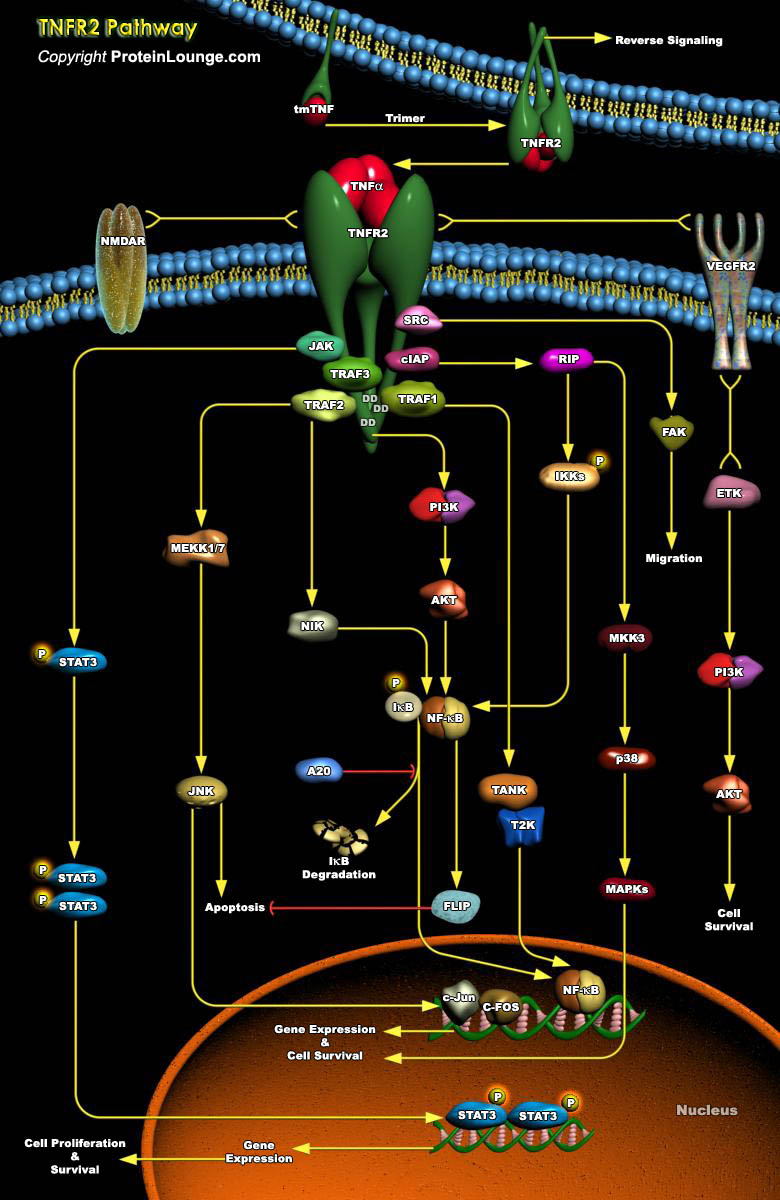
Tumor necrosis factor (TNF) is a pro-inflammatory cytokine with the capacity to induce apoptosis. It is enriched in the tumor microenvironment, promotes tumor growth and subverts innate immune responses to cancer cells. TNF is the best studied member of the TNF superfamily. TNF-alpha can bind to two related receptors, TNF receptors 1 and 2 (TNFR1 and TNFR2), which are also used by other, similar ligands. By binding to TNFR1 and TNFR2, TNF activates distinct signaling pathways important for cell proliferation, cell death and immune responses (Ref.1 and 2). TNFR2 is typically restricted to certain subpopulations of immune cells such as CD4+ or CD8+ T cells and a few other cell types such as oligodendrocytes and endothelial cells (Ref.3).TNFR2 signaling has significant[..]
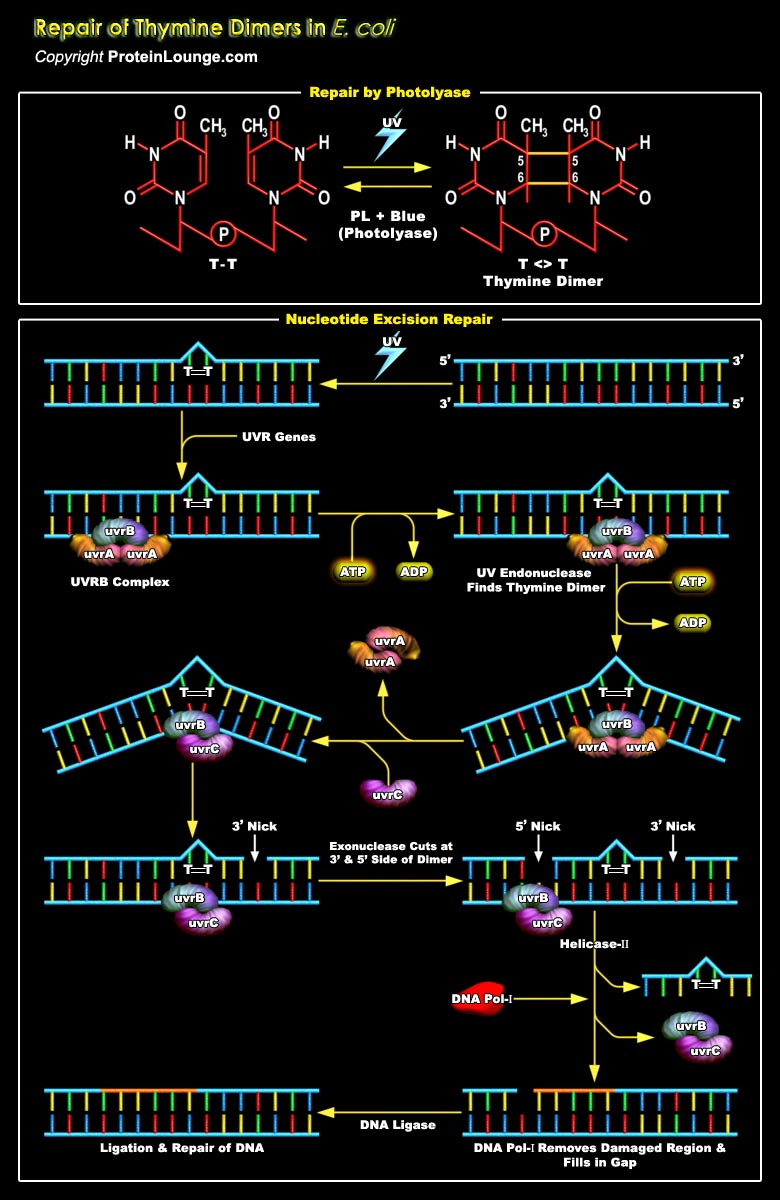
UV radiation induces two of the most abundant mutagenic and cytotoxic DNA lesions such as CPD (Cyclobutane-Pyrimidine Dimers) or 6-4PPs (6-4 Pyrimidine Pyrimidone). The most common covalently linked adjoining pyrimidines are TT(Thymine dimers), T-C (Thymine-Cytosine dimers) and C-C (Cytosine-Cytosine dimers). T-T dimers cause kinks in the DNA strand that prevent both replication and transcription of that part of the DNA. Because they block DNA replication (and therefore prevent cells from reproducing), T-T dimers and other forms of UV damage cannot be inherited, and thus do not constitute mutations. Such kinds of DNA damage are known as premutational lesions because they prevent both transcription and replication of the genes in which they are present and[..]
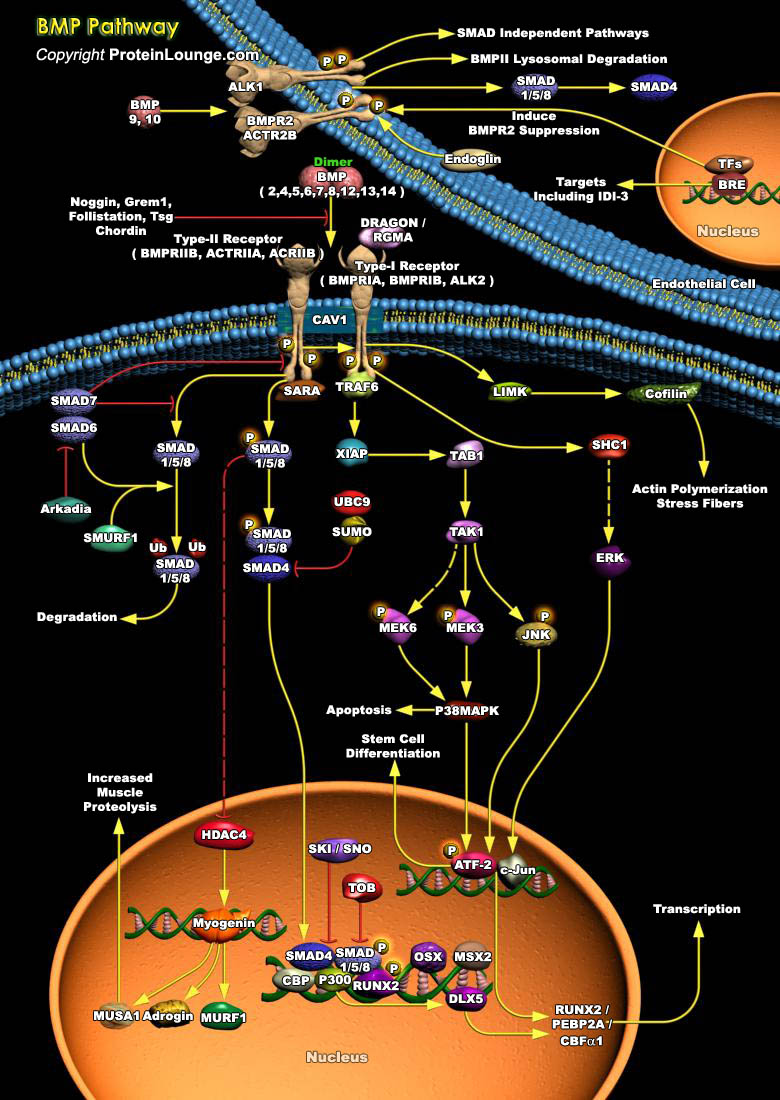
BMPs (Bone morphogenetic proteins) are the members of the transforming growth factor-beta superfamily of secreted signaling molecules [Ref.1]. Transduction of BMP signal involves two types of transmembrane serine/threonine kinase receptors: type I and type II [Ref.1]. BMP2, BMP4, BMP5,BMP6, BMP7, BMP8, BMP9, BMP10, BMP12, BMP13 and BMP14 (Bone morphogenetic protein 2, 4, 5, 6 ,7, 8, 9, 10, 12, 13 and 14) can bind to three type I receptors: BMPR1A (Bone morphogenetic protein receptor, type IA), BMPR1B (Bone morphogenetic protein receptor, type IB) and ALK-2 (Activin A receptor, type I) and two type II receptor, BMPR2A (Bone morphogenetic protein receptor type IIA) and ACTRIIA [Ref.1]. Various antagonists such as, Noggin, Tsg (twisted gastrulation), GREM1, Follistatin[..]
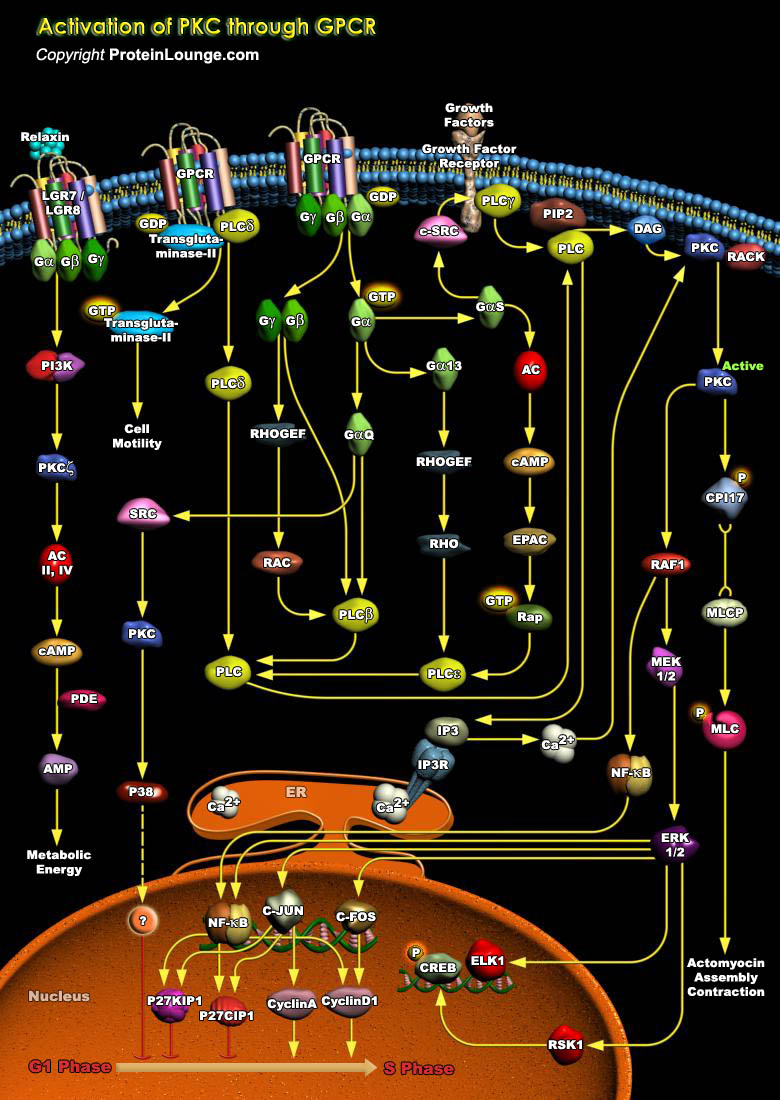
PKC (Protein Kinase-C) is a cyclic nucleotide-independent enzyme that phosphorylates serine and threonine residues in many target proteins. PKC plays a pivotal role in mediating cellular responses to extracellular stimuli involved in proliferation, differentiation, apoptosis, and exocytotic release in a number of non-neuronal systems such as Islet cells, Chromaffin cells and Paramecium. PKC has also been implicated in phosphorylation of several neuronal proteins, which are thought to regulate neurotransmitter release and establish long-term potentiation in memory formation. PKC is not a single enzyme but a family of serine/threonine kinases. At least eleven closely related PKC isozymes have been reported that differ in their structure, biochemical properties, tissue[..]
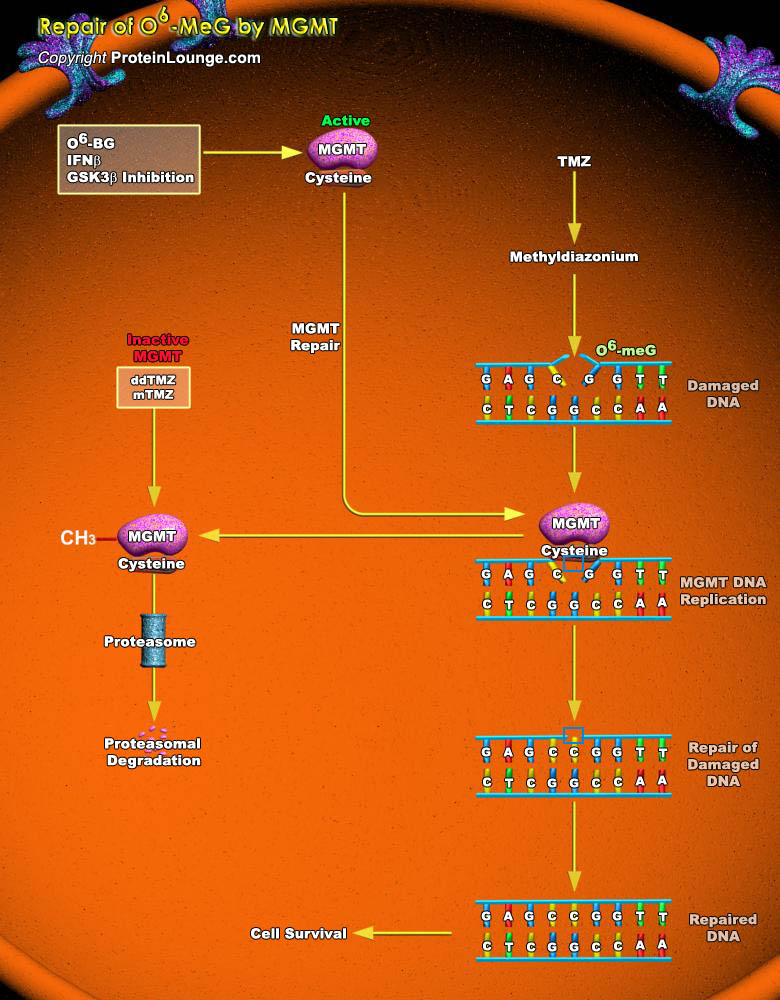
Alkylating agents are compounds that cause cytotoxic DNA damage as well as collateral mutagenic damage. They work by adding an alkyl group to the guanine base of the DNA molecule and cause breakage of DNA strands. Methylation at the guanine O6 position forms the greatest promutagenic and lethal toxic DNA lesion. O(6)-methylguanine (O6-meG) is formed in DNA by alkylation of the oxygen atom of guanine, most often by N-nitroso compounds (NOC) and sometimes due to methylation by other compounds such as endogenous S-adenosyl methionine. Several repair pathways like direct DNA damage reversal, base excision repair (BER) and mismatch repair (MMR), respond to alkylation damage to defend against alkylation-induced cell death or mutation. Direct reversal repair eliminates[..]
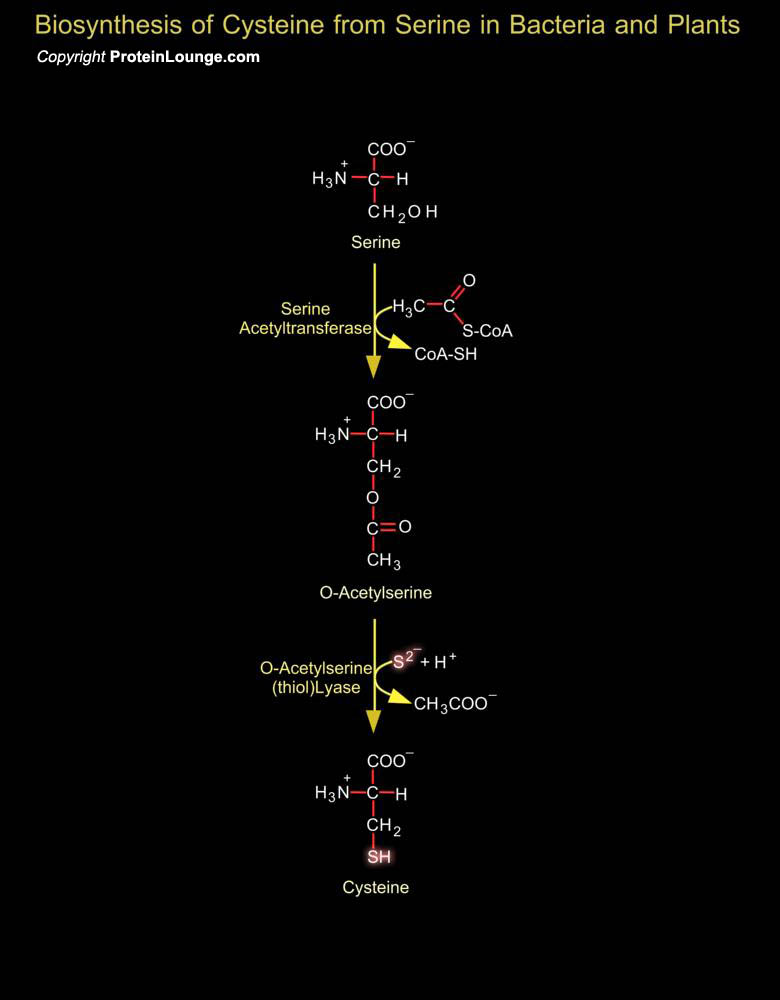
Inorganic sulfur in the environment (primarily sulfate, but also sulfur, and sulfite) must undergo fixation to be utilized by organisms. The fixation of sulfate is largely confined to plants and bacteria and biosynthesis of cysteine represents the final step of sulfate assimilation in these organisms. Fixation begins with the formation of PAPS (3'-Phosphoadenosine-5'-Phosphosulfate). PAPS is an activated sulfate compound and an intermediate in all organisms for sulfate esterification, such as the synthesis of chondroitin sulfate. It is formed in a two-step reaction from sulfate ion and two molecules of ATP. In plants, the main pathway of sulfate reduction is via APS (Adenosine-5'-Phosphosulfate) rather than PAPS (i.e. APS can be utilized directly, without[..]
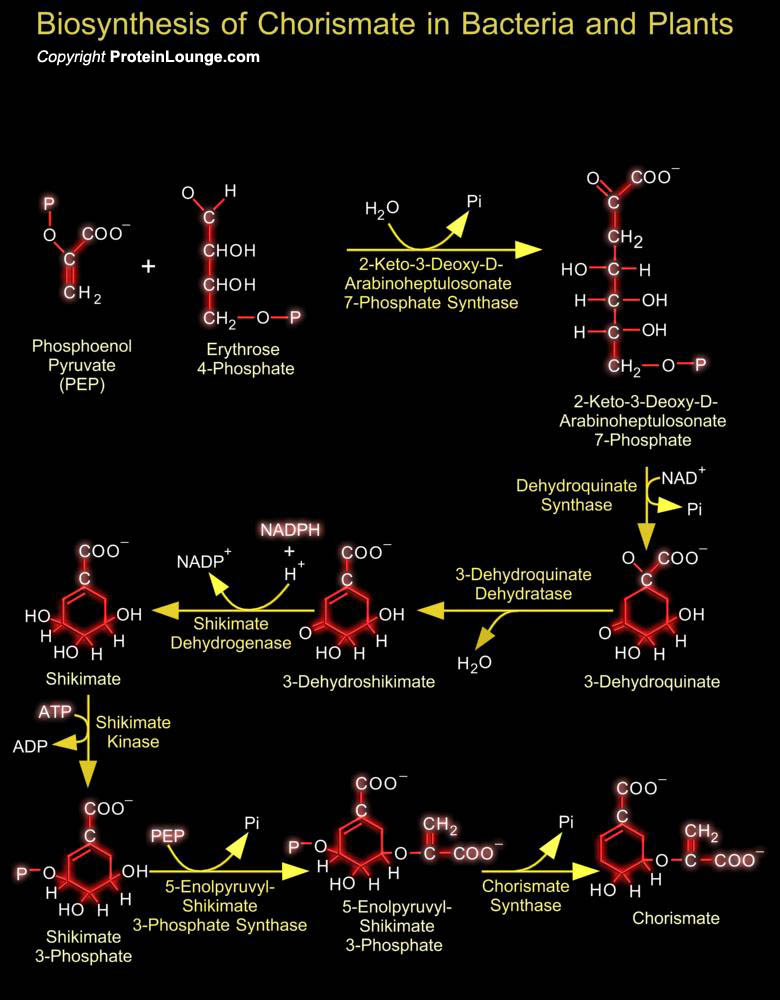
In micro-organisms and plants the biosynthesis of aromatic compounds proceeds via the common seven-step aromatic or shikimate pathway to the branch point intermediate chorismate. This intermediate is subsequently converted to the three aromatic amino acids via specific terminal pathways. Many other aromatic compounds are derived either partially or entirely from chorismate or from other pathway intermediates or end products.The first step in the biosynthesis of Chorismate involves PEP (Phosphoenolpyruvate) from glycolysis and E-4-P (Erythrose 4-Phosphate) from the Pentose Phosphate Cycle. These two precursors are condensed and then cyclized to form 3-Dehydroquinate, followed by removal of a water and a reduction step to produce Shikimate. The first cyclic intermediate[..]

