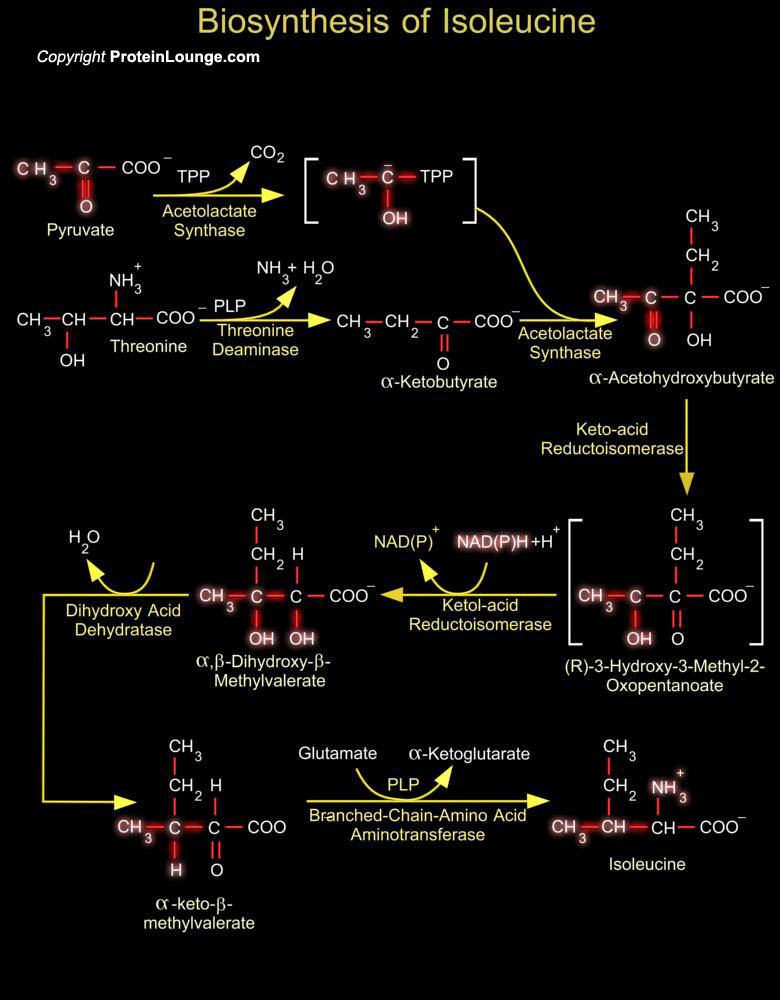
Isoleucine encoded by the codons AUU, AUC, and AUA used in the biosynthesis of proteins. It is a α-amino acid that contains an α-amino group, an α-carboxylic acid group, and a hydrocarbon side chain. It is classified as a non-polar, uncharged (at physiological pH), branched-chain, aliphatic amino acid. Isoleucine is essential in humans, meaning the body cannot synthesize it, and must be ingested in our diet. Isoleucine is synthesized from pyruvate employing leucine biosynthesis enzymes in other organisms such as bacteria (Ref.1).The biosynthesis pathway of L-valine and L-isoleucine from L-threonine is a part of the super pathway of branched amino acid biosynthesis that also generates L-leucine. The first enzyme involved in the pathways[..]
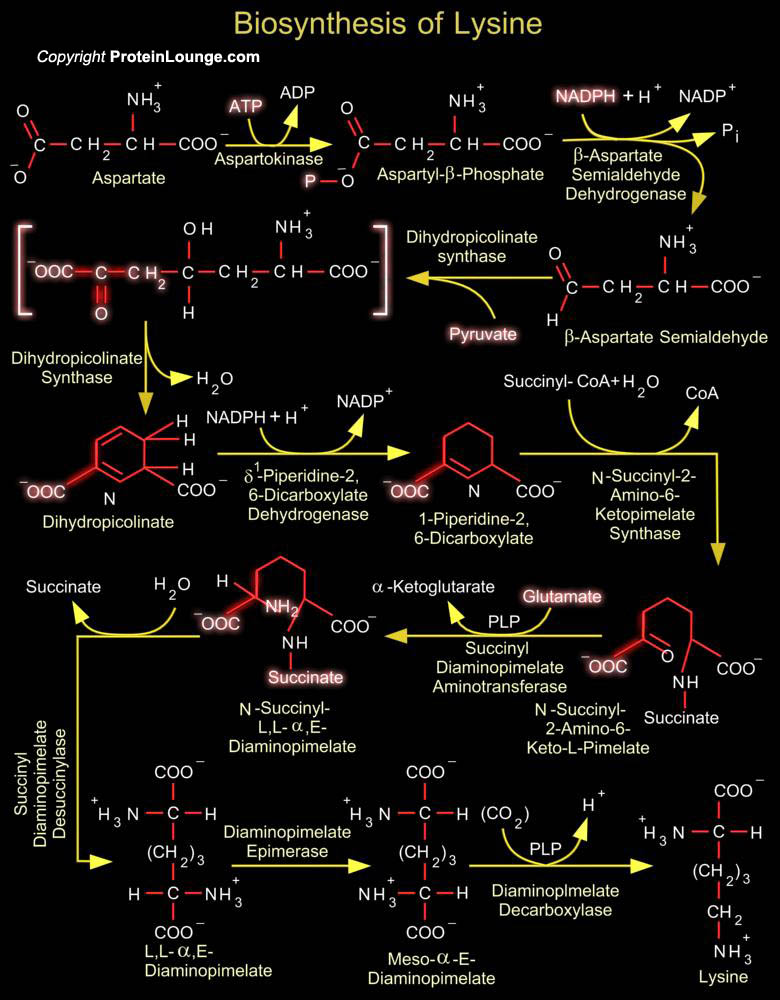
The amino acid L-lysine is synthesized by plants and microorganisms by two different pathways, one proceeding via Diaminopimelate and the other via Alpha-Aminoadipate. Humans, however, cannot synthesise the compound and so it is an essential part of their diet. The Diaminopimelate pathway operates in bacteria, lower fungi and green plants. This pathway is the source of the lysine and Diaminopimelate incorporated into bacterial cell wall peptidoglycan and therefore there has been extensive investigation of its enzymes with a view to the development of new antibacterial agents. In Euglenoids and higher fungi lysine biosynthesis occurs via the Homocitrate-Aminoadipate pathway. An intermediate on this pathway (Alpha-Aminoadipic acid) is necessary for Beta-lactam antibiotic[..]
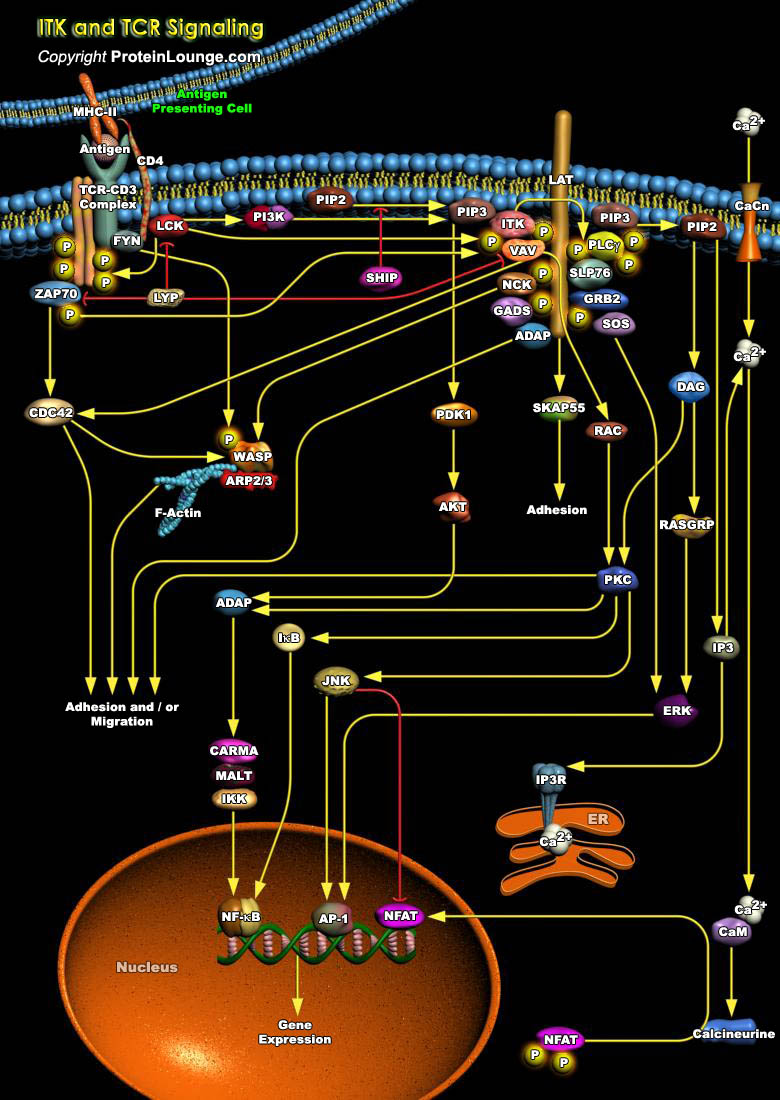
Development of a proper immune system requires the selection of lymphocytes expressing a useful repertoire of antigen receptors that can respond to foreign or dangerous antigens but not to self. For T-Cells developing in the thymus, these selection processes include both positive and negative selection of immature CD4 and CD8 cells, helping shape the mature T-Cell repertoire. These processes are regulated in large part through the interactions between the TCR (T-Cell Antigen Receptor) expressed on a given thymocyte and peptides presented in the context of either Class-II or Class-I MHC molecules. In T-Cells, at least three Tec Kinases are expressed: Tec, RLK (Resting Lymphocyte Kinase)/TXK and ITK (Inducible T-Cell Kinase), which have expression primarily restricted to[..]
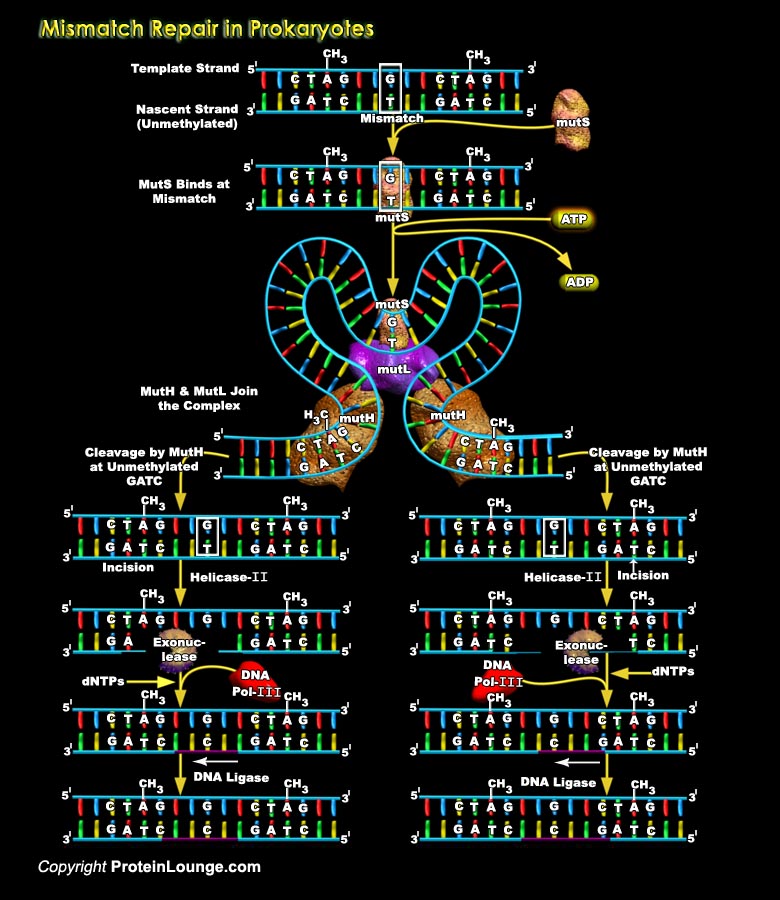
The integrity of genetic information depends on the fidelity of DNA replication and on the efficiency of several different DNA repair processes. Among many types of DNA repair, the general MMR (DNA Mismatch Repair) pathway is responsible for correcting base substitution mismatches which is generated during DNA replication in organisms from bacteria to mammals. The MMR system improves the fidelity of DNA synthesis by 100-1000 fold. The MMR also corrects IDLs (Insertion-Deletion Loops) which may occur during replication and recombination of DNA. MMR also able to correct DNA damages caused by internal or external sources. Inactivation or deterioration of this pathway has been linked to a variety of cancers, most notably Hereditary Non-Polyposis Colorectal Cancer. MMR can[..]
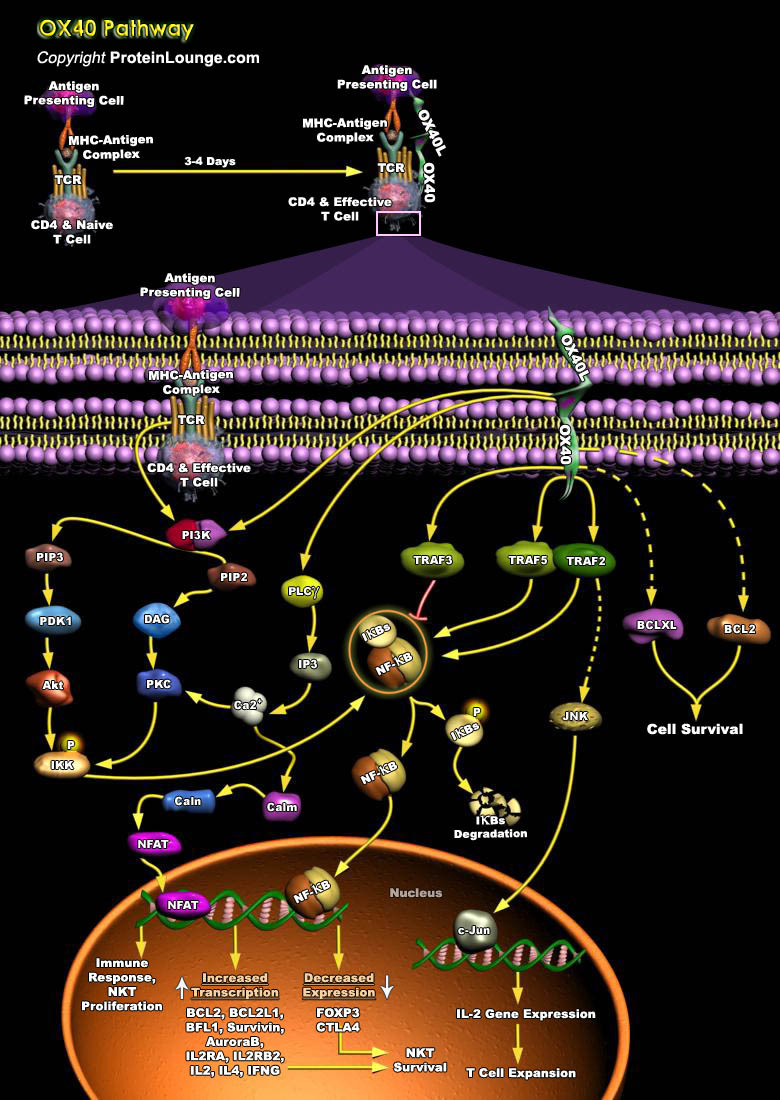
OX40 is an approximately 50-kD transmembrane glycoprotein of 249 amino acids, with a 49 amino acid cytoplasmic tail and a 186 amino acid extracellular region. It is a member of the TNFR (tumor necrosis factor receptor) superfamily and has three complete and one truncated cysteine-rich domains. It is a T-Cell activator that is believed to promote the survival (and perhaps prolong the immune response) of CD4+ T cells at sites of inflammation (Ref.1). OX40’s ligand (OX40L, also known as gp34, CD252, TNFSF4), a member of the TNF (Tumor Necrosis Factor) super family, is a 32 kDa protein, which has about 183 aa residue glycosylated polypeptide that consists of a 23 amino acid cytoplasmic tail and a 133 amino acid extracellular domain. It is a type II glycoprotein that[..]
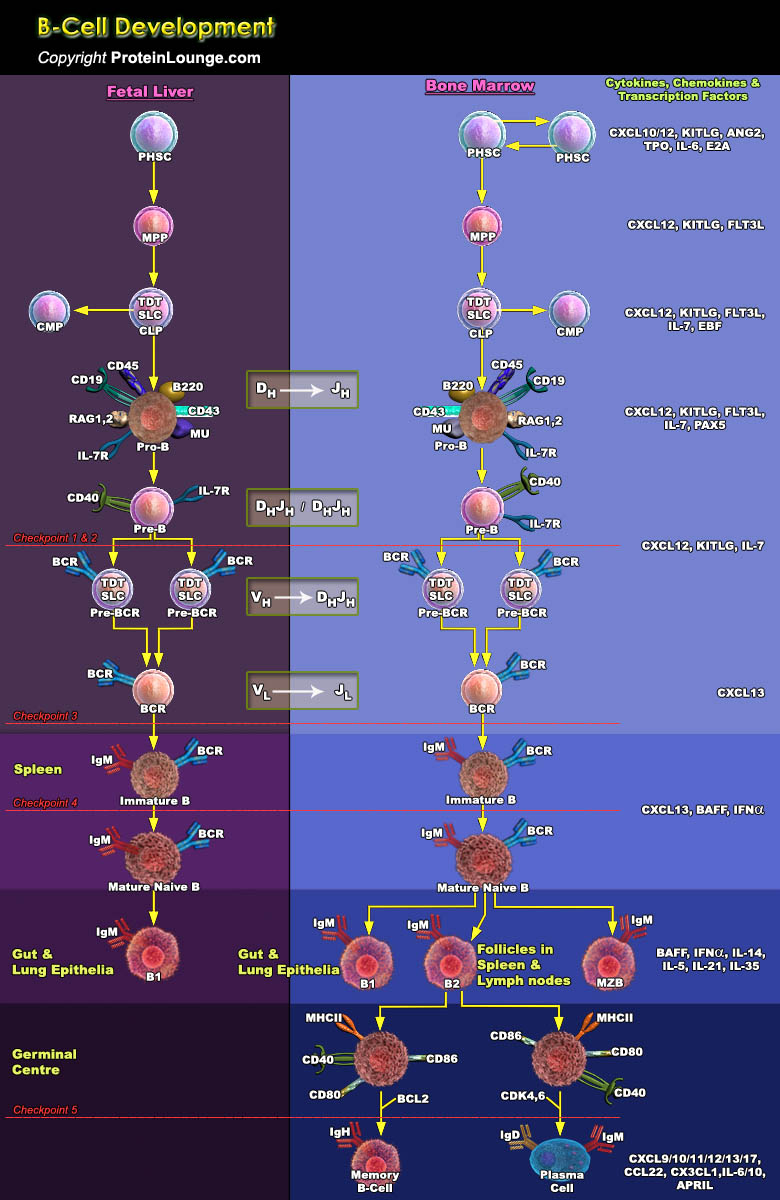
B lymphoid cells are essential for the humoral immune response by producing a diverse range of antigen-specific antibodies. Antibody-mediated immunity is provided by two distinctive B cell lineages that diverge early in life. The better-known conventional, or B2, B cells provide adaptive immunity by producing high-affinity pathogen-specific antibodies, typically in a T cell-dependent manner. The pool of B2 cells is constantly replenished from hematopoietic stem and progenitor cells in the bone marrow [Ref.1]. B1a cells provide innate-like immunity by producing “natural” low-affinity, broad-specificity antibodies against a wide range of overlapping antigens, including self-antigens. In contrast to B2 cells, the pool of B1a cells is largely established at[..]
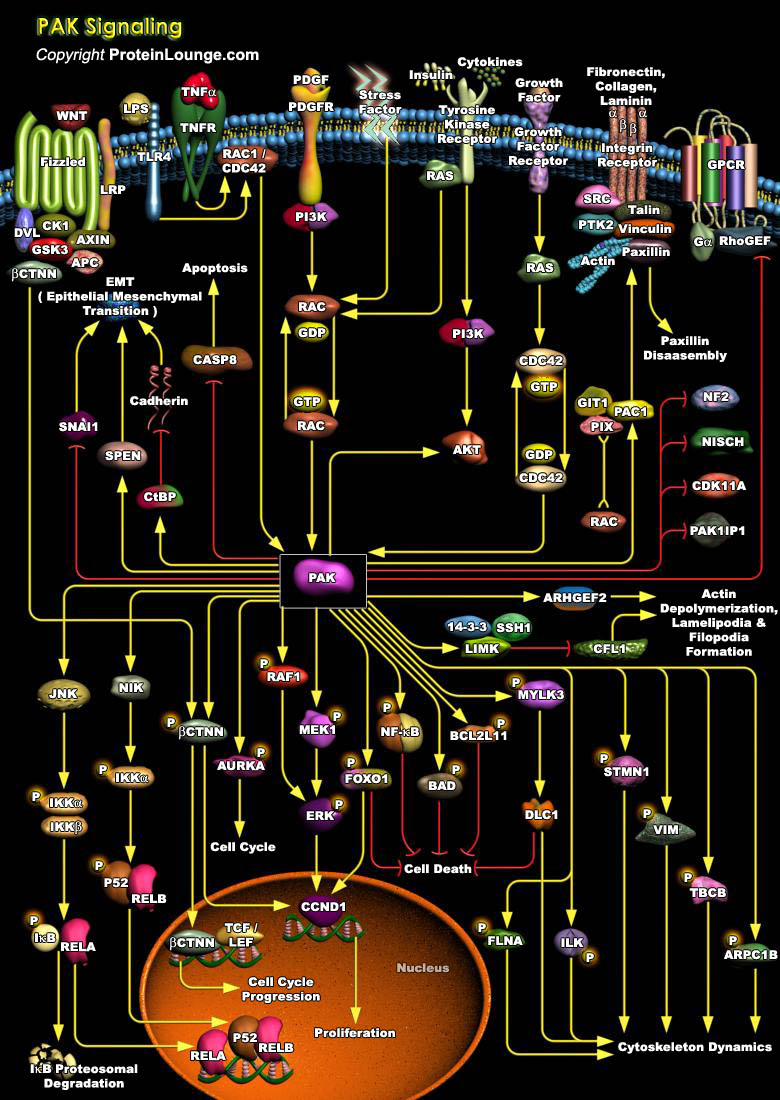
p21-activated kinase (PAK) family of serine/threonine protein kinases are downstream effectors of the Rho family of GTPases (Rac and Cdc42). PAKs are found in most eukaryotes and play an evolutionarily conserved role in many cellular processes like cell proliferation, survival, gene transcription, transformation, and cytoskeletal remodeling (Ref.1). There are six PAK isoforms identified in humans that share a conserved catalytic domain located at the C-terminus. They have been classified into two groups (I and II) according to the similarity of their catalytic domains, and regulatory mechanisms. The C-terminal kinase catalytic domain is similar among all human PAKs where as the N-terminal regions are more variable. Group I PAKs consists of (isoforms 1, 2 and 3) where[..]
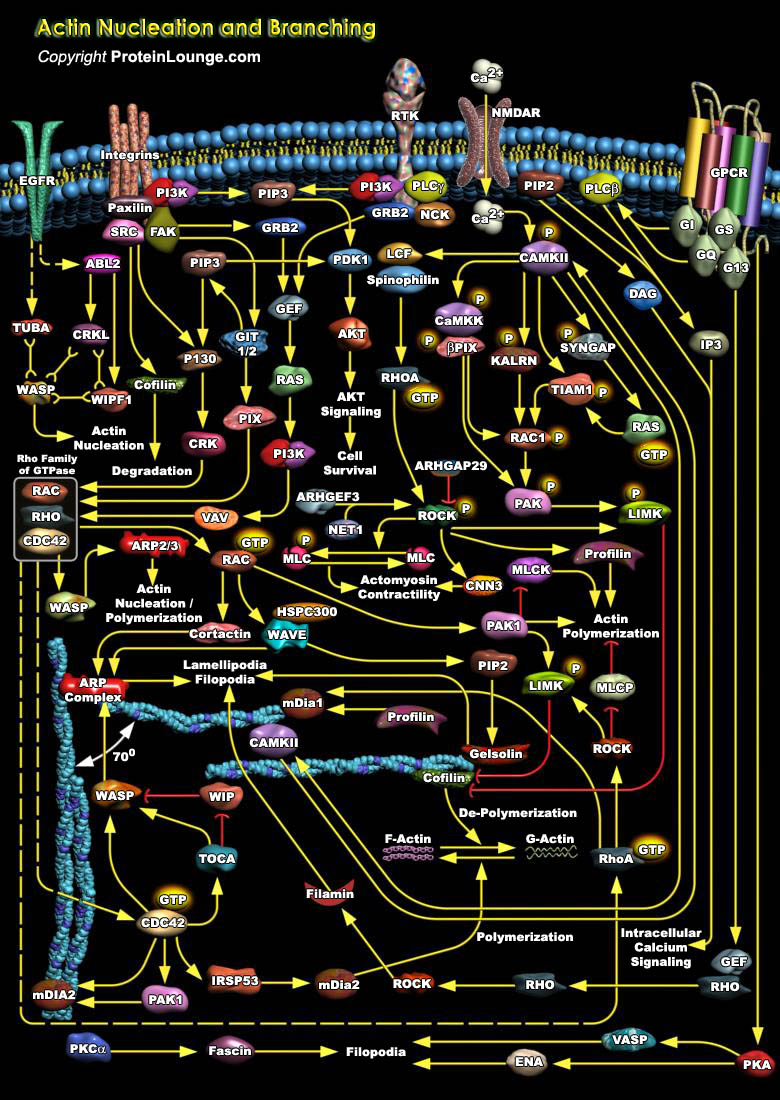
The actin family is a diverse and evolutionarily ancient group of proteins that provide the supportive framework to the three-dimensional structure of eukaryotic cells. It provides the forces that enable the cell to adopt a variety of shapes and to undertake directed movements. Certain cell types, such as polymorphonuclear leukocytes, monocyte/macrophages, and metastatic cells, are able to move rapidly through tissues and these movements are mediated by the actin cytoskeleton (Ref.1). An important property of actin is its ability to produce movement in the absence of motor proteins. At the cell membrane actin microfilament assembly protrudes the membrane forward producing the ruffling membranes in actively moving cells. These microfilaments also play a passive[..]
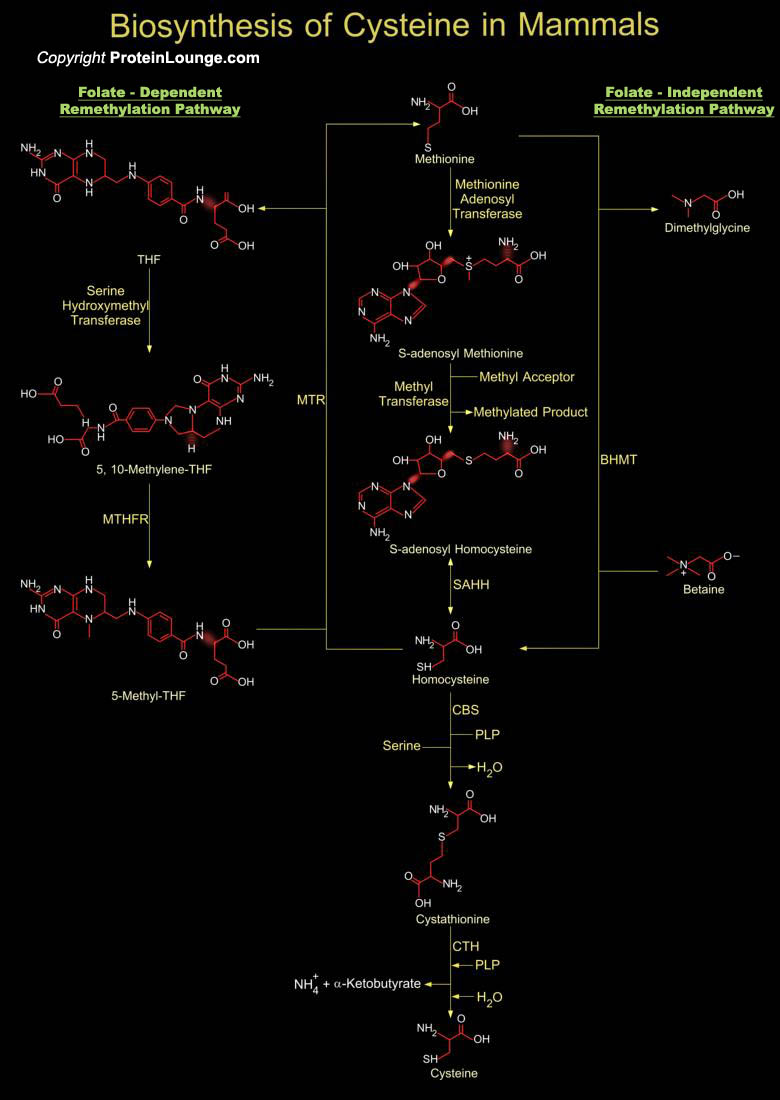
Cysteine, a sulfur-containing amino acid, is indispensable for the survival of virtually all living organisms, from bacteria to higher eukaryotes. This amino acid is implicated in several processes, including the stability, structure, regulation of catalytic activity, and post-translational modification of various proteins. Due to the ability of its thiol group to undergo redox reactions, Cysteine forms the basic building block of all thiol antioxidants, acting as a direct antioxidant and also as a precursor for the biosynthesis of glutathione, trypanothione, or ovothiol. In addition, cysteine is also essential for the synthesis of biomolecules, including coenzyme A, hypotaurine, taurine, and ubiquitous iron-sulphur (Fe-S) clusters, which are involved in electron[..]
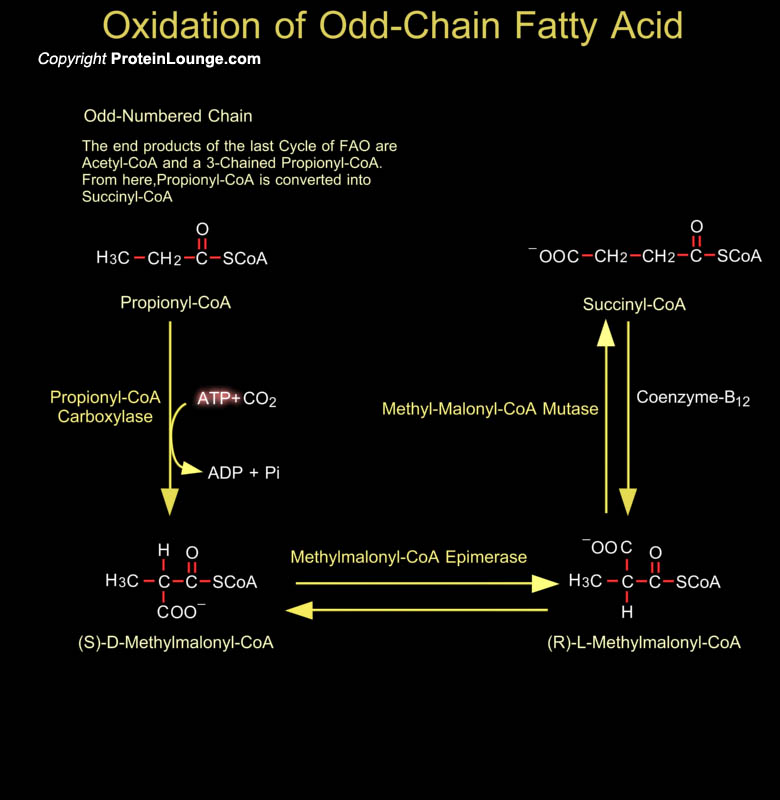
Most fatty acids have even number of carbon atoms and are therefore completely converted to Acetyl-CoA. Some plants and marine organisms, however, synthesize fatty acids with an odd number of carbon atoms. The final round of Beta-Oxidation of these fatty acids forms Propionyl-CoA, which is converted to Succinyl-CoA for entry into the Citric Acid Cycle. Propionyl-CoA can be derived from breakdown of certain amino acids (Isoleucine, Valine and Methionine), but in mammalian cells these pathways exist in mitochondria. Bacteria in the ruminant animal digestive system provide a source of propionate which eventually gets incorporated as odd-numbered fatty acids in milk fats, and this is the major source for human metabolism. The conversion of[..]

Ras is a membrane-associated guanine nucleotide-binding protein that is normally activated in response to the binding of extracellular signals, such as growth factors, RTKs (Receptor Tyrosine Kinases), TCR (T-Cell Receptors) and PMA (Phorbol-12 Myristate-13 Acetate). Ras signaling affects many cellular functions, which includes cell proliferation, apoptosis, migration, fate specification, and differentiation. Ras acts as a binary signal switch cycling between ON and OFF states, which are characterized in terms of a small molecule, a guanine nucleotide, bound to the protein. In the resting cell, Ras is tightly bound to GDP (Guanosine Diphosphate), which is exchanged for GTP (Guanosine Triphosphate) upon binding of extracellular stimuli to cell membrane receptors. In the[..]
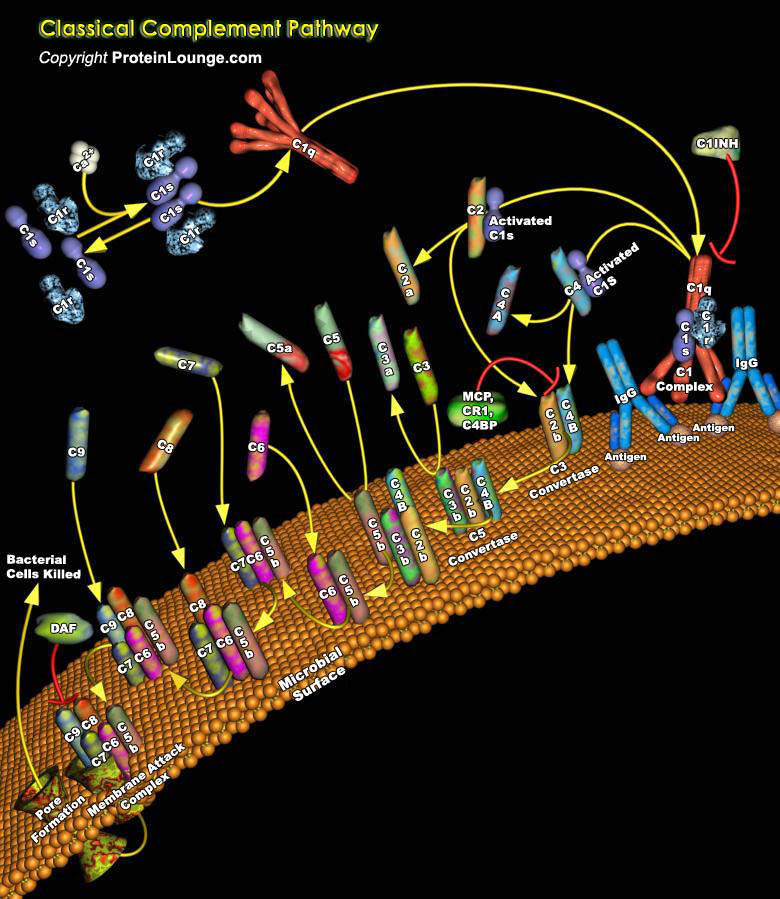
Complement is a system of circulating enzymes that is part of the body's response to illness or injury. The complement system plays an essential role in host defence against infectious agents and in the inflammatory process. It consists of about thirty plasma proteins that function either as enzymes or as binding proteins. In addition to these plasma proteins, the complement system includes multiple distinct cell-surface receptors that exhibit specificity for the physiological fragments of complement proteins and that occur on inflammatory cells and cells of the immune system. There are also several regulatory membrane proteins that function to prevent autologous complement activation and protect host cells from accidental complement attack. The complement system can[..]

