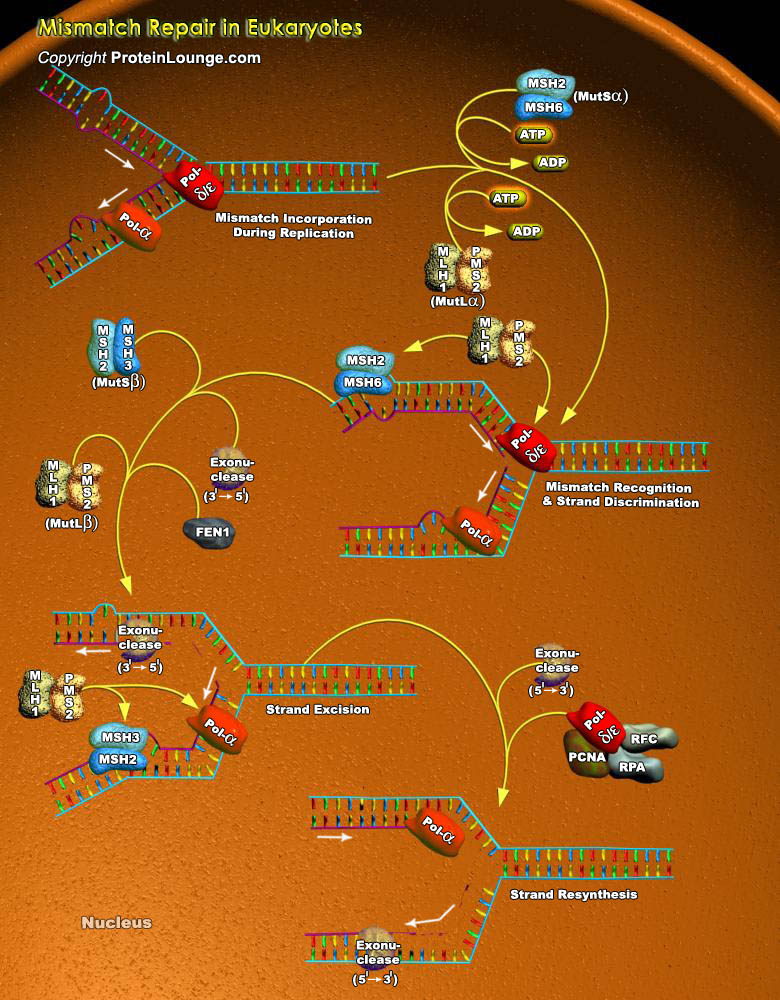
The major DNA Repair mechanisms take advantage of the facts that DNA is double-stranded and the same information is present in both strands. Consequently, in cases where damage is present in just one strand, the damage can be accurately repaired by cutting it out (excision) and replacing it with new DNA synthesized using the complementary strand as template. All organisms, prokaryotic and eukaryotic, employ at least three excision mechanisms: Mismatch Repair, Base Excision Repair, And Nucleotide Excision Repair. Mismatch repair successfully meets a number of challenges. First, it recognizes a wide spectrum of rare mismatches, embedded in millions of correctly base-pairing nucleotides. Second, mismatch repair unequivocally discriminates between a correct nucleotide in[..]
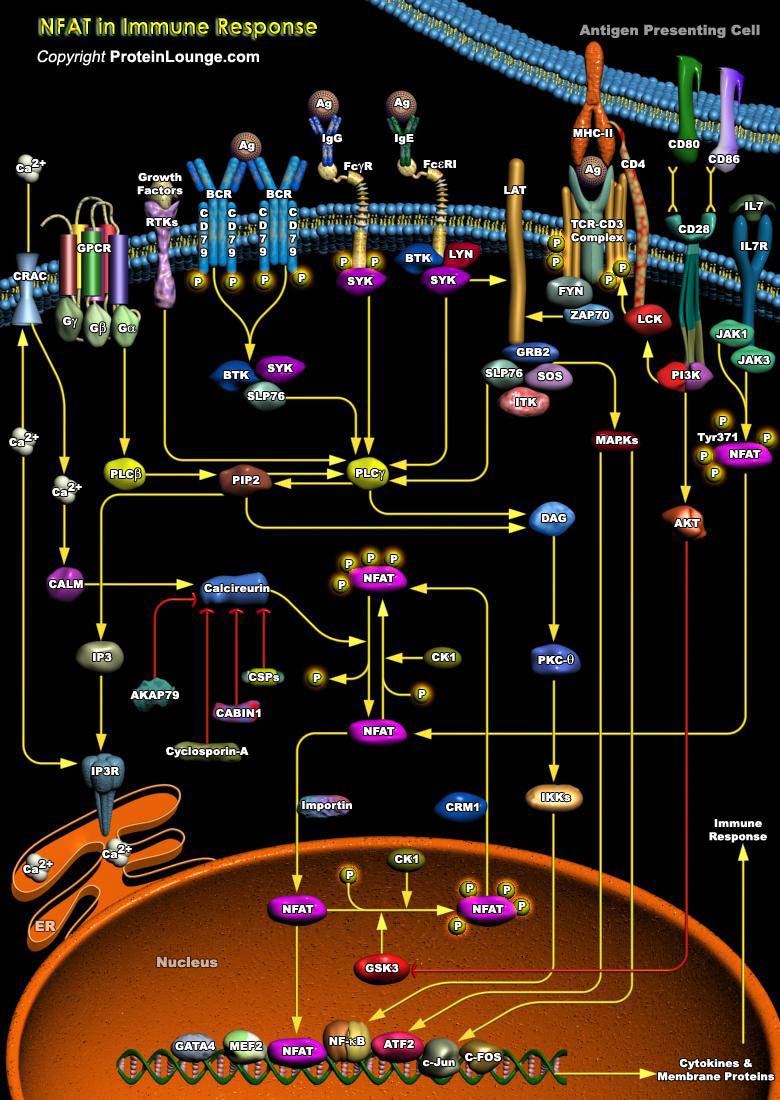
Antigenic stimulation of lymphocytes and other cells of the immune system initiates a complex series of intracellular signal transduction pathways that lead to the expression of a panel of immunoregulatory genes, whose function is critical to the initiation and coordination of the immune response. NFATs (Nuclear Factors of Activated T-Cells), a family of transcription factors expressed both inside and outside of diverse cell types of the immune system, play a pivotal role in the process. Originally described in T-Cells, NFATs have now been implicated in the activation of mast cells, B-Cells, NK (Natural Killer) cells, monocytes, and play a key role in the expression of a number of immunologically important genes, including a wide array of cytokines: IL-2, IL-3,[..]
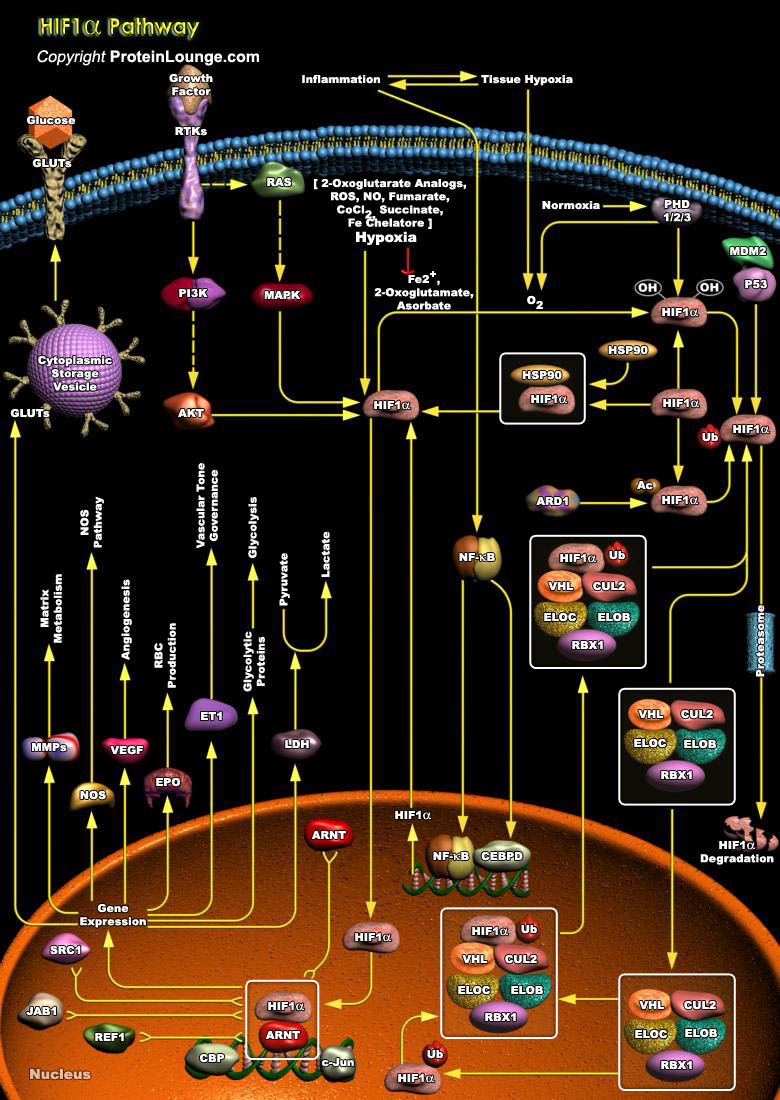
The cellular response to O2 (oxygen) is a central process in animal cells and figures prominently in the pathophysiology of several diseases, including cancer, cardiovascular disease, and stroke. This process is coordinated by the HIF (Hypoxia-Inducible Factor) and its regulator, the pVHL (Von Hippel-Lindau tumor suppressor protein). HIF1 is a basic helix-loop-helix transcription factor that transactivates genes encoding proteins that participate in homeostatic responses to hypoxia. It induces expression of proteins controlling glucose metabolism, cell proliferation, and vascularization. Several genes involved in cellular differentiation are directly or indirectly regulated by hypoxia. These include Epo (Erythropoietin), LDHA (Lactate Dehydrogenase-A), ET1[..]
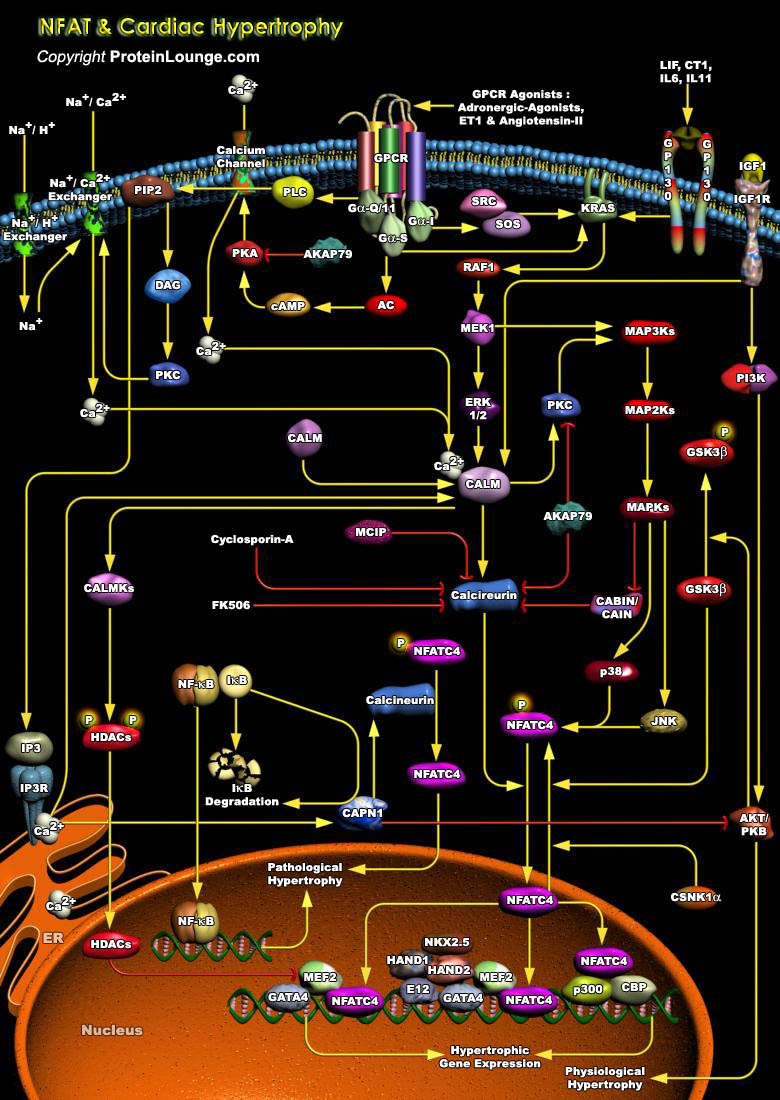
Cardiac failure, one of the largest health care burdens in the United States and other developed countries is often associated with prolonged and maladaptive cardiac hypertrophy, defined as a compensatory mechanism of the heart that helps to maintain cardiac output during pathological states with sustained increases in hemodynamic load (Ref.1). As cardiomyocytes lose the ability to divide soon after birth, cardiac hypertrophy offers an important adaptive response in vivo that allows the organism to maintain or increase its cardiac output. The adult myocardium responds to a wide array of intrinsic and extrinsic stimuli, including hypertension, myocardial infarction, cardiac arrhythmias, valvular disease, endocrine disorders, increased workload, injury, and[..]
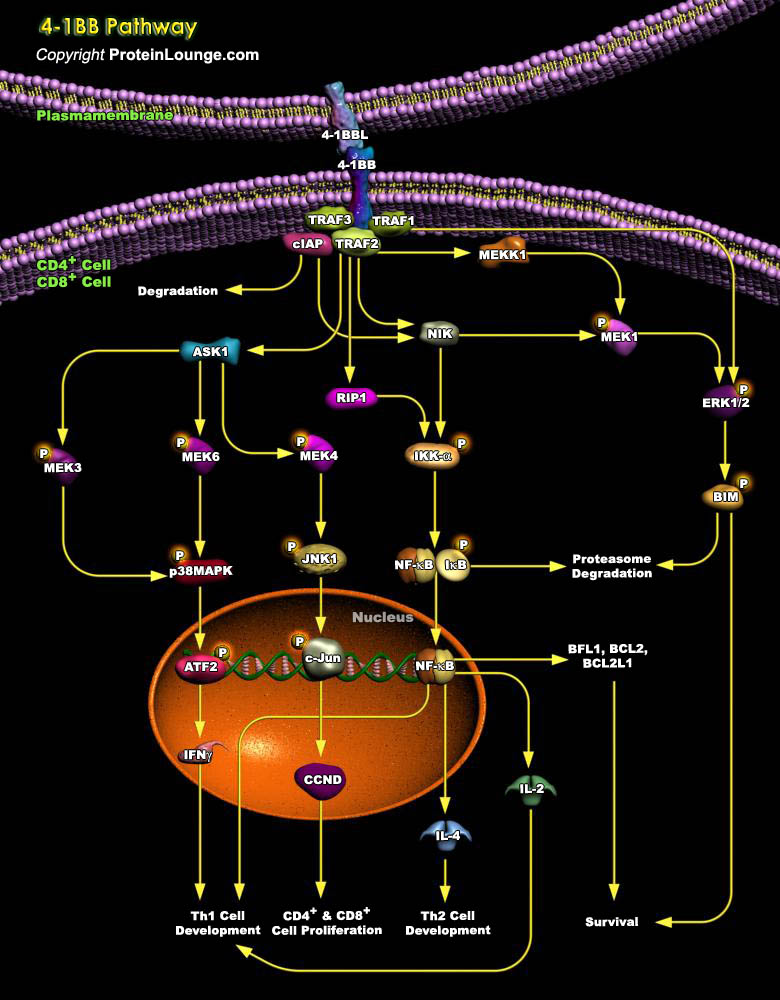
4-1BB is an inducible T cell surface receptor belonging to the tumor necrosis factor receptor super family. It presents on the surfaces of activated CD4+ and CD8+ T cells, monocytes and B lymphocytes. 4-1BB signaling is activated by binding to its high-affinity ligand 4-1BBL which is expressed on the surface of antigen-presenting cell. 4-1BB signaling promotes cell growth, T cell differentiation and survival of immune cells (Ref.1&2).4-1BB mediates its action via association with TRAF1, TRAF2 and TRAF3. 4-1BB binding to TRAF2 and TRAF3/ cIAP complex leads to activation of the NIK. NIK activates NF-kB via the IKK-alpha/ NFKBIA pathway (Ref.2, 3, 4&5).NF-kB induces transcription of Bcl-XL, Bcl-2 and BFL1, and thus promotes survival of immune cells (Ref.6,[..]
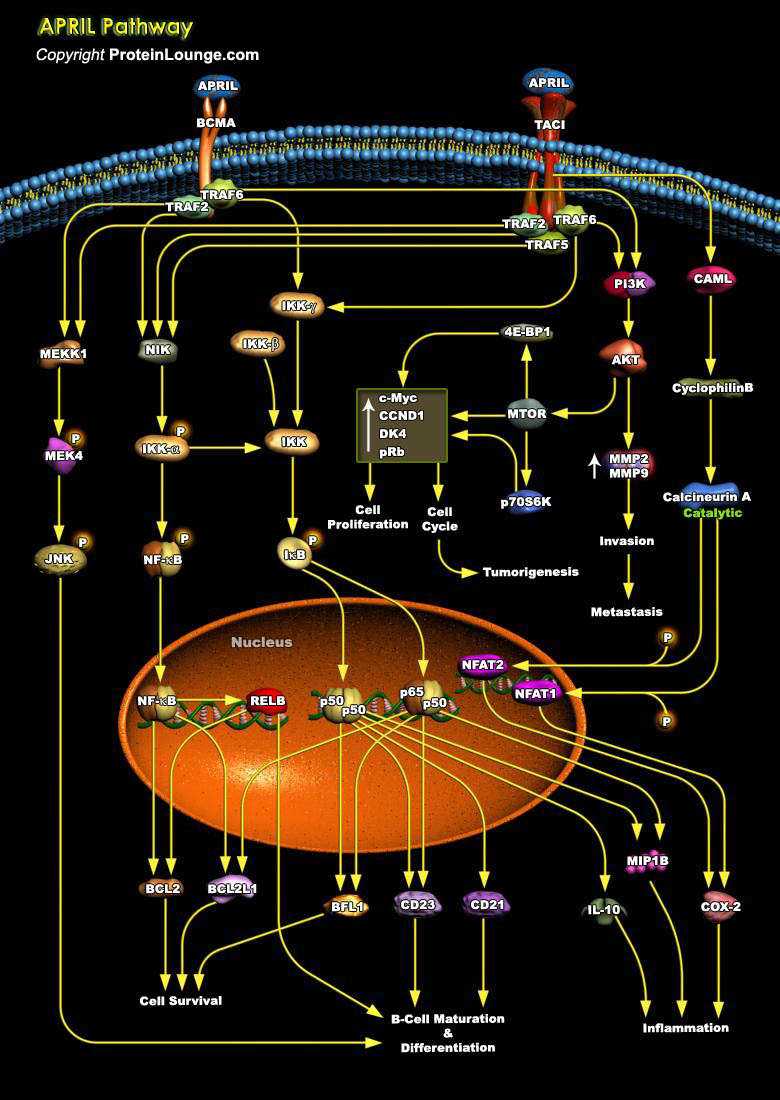
APRIL (a proliferation-inducing ligand, also known as TRDL-1, TALL-2, and TNFSF13), is a member of the TNF (tumor necrosis factor) superfamily, with homologous structure and function to several other cytokines in this family. It is a cytokine which is over-expressed by transformed cells and could stimulate cellular proliferation (Ref.1) It has two receptors i.e BCMA and TACI (Tumor necrosis factor receptor superfamily members 17 and 13B) that play important roles in the B-cell and T-cell arms of the immune system (Ref.2 & 3). One of the most important APRIL-induced mechanism is an activation of NF-kB (Nuclear factors of kappa light polypeptide in B-cells) signaling cascades. Activation and nuclear translocation of NF-kB proteins can occur by one of two pathways:[..]
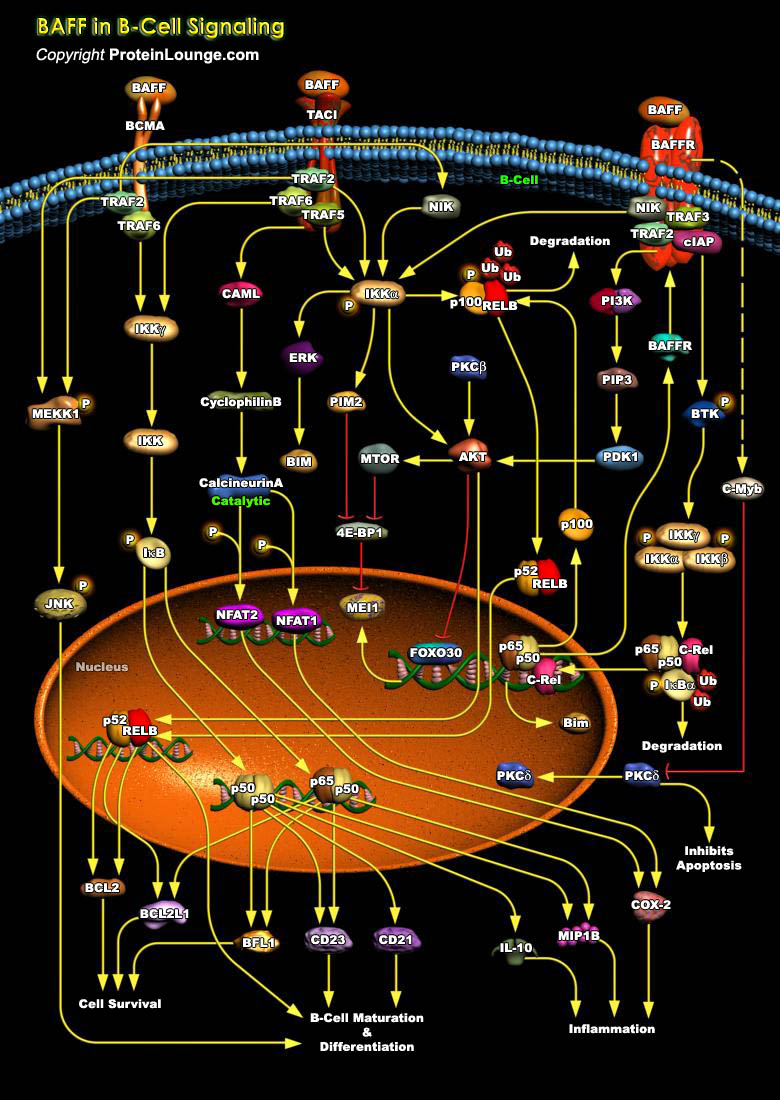
BAFF (B-cell activating factor), is a member of the TNF (tumor necrosis factor) ligand family that plays important roles in B-cell homeostasis, tolerance, and malignancy (Ref.1). It is also known as BLyS (B lymphocyte stimulator) protein, TALL-1 (TNF and apoptosis ligand-related leukocyte-expressed ligand-1), zTNF4, CD257, TNFSF13B, TNFS20 (TNF superfamily member) and THANK (TNF homolog that activates apoptosis, NF-B and c-Jun NH2-terminal kinase). It functions through three receptors i.e. TACI, BCMA and BAFFR (BAFF-receptor) (Ref.1). Interaction of BAFF with BAFFR leads to recruitment of TRAF3 to the receptor and it is subsequently degraded by the combined actions of TRAF2 and cIAP1/2 (Ref.2). Lack of TRAF3 deactivates the TRAF/cIAP complex, releasing NIK and[..]

Protein synthesis in eukaryotic organisms is a complex process that requires cooperation among a large number of polypeptides including ribosomal proteins, modification of enzymes, and ribosome-associated translation factors. The initiation phase of protein synthesis, during which ribosomes select mRNAs to be translated and identify the translational start site, requires a set of eIFs (eukaryotic Translation Initiation Factors), many of which are comprised of multiple polypeptide subunits. EIF2 (Eukaryotic Initiation Factor-2) is a GTP (Guanosine Triphosphate)-binding protein that escorts the initiation-specific form of Met-tRNA (Met-tRNAi) onto the ribosome. It is composed of 3 non-identical subunits, alpha (36 kD), beta (38 kD), and gamma (52 kD). cDNAs for each of[..]
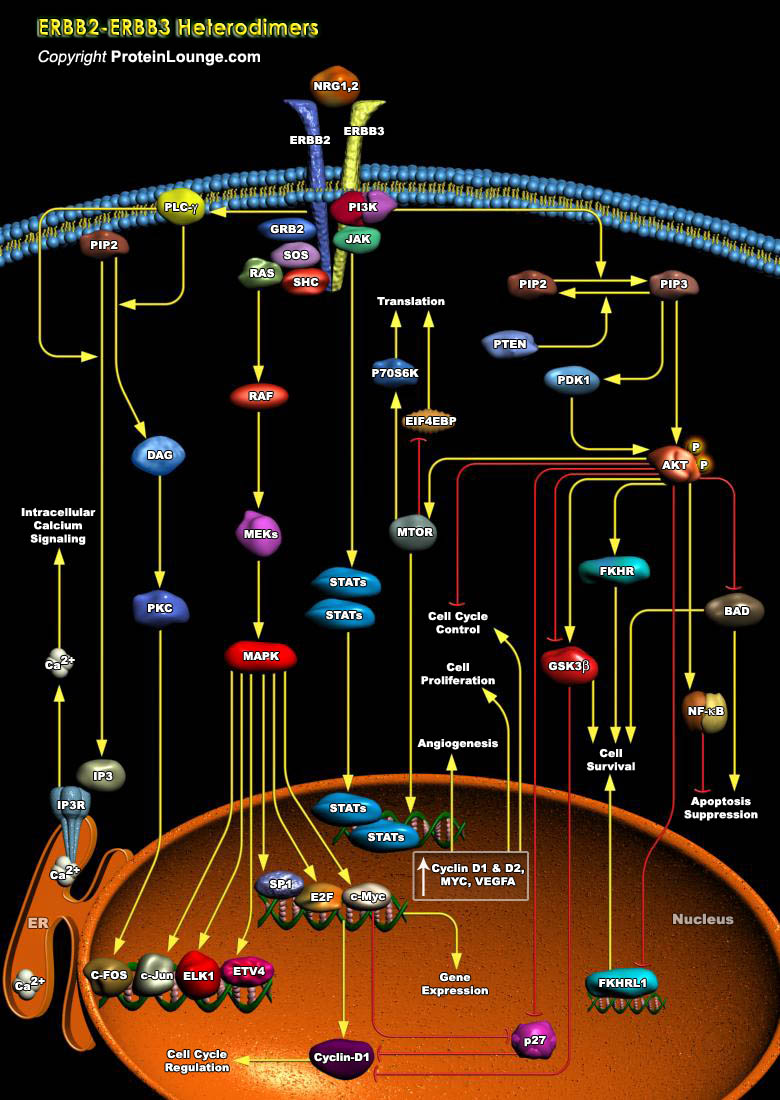
The ERBB (Erythroblastic Leukemia Viral Oncogene Homolog) family of transmembrane RTKs (Receptor Tyrosine Kinases) plays an important role in the pathogenesis of many cancers. This family is comprised of four members EGFR (Epidermal Growth Factor Receptor), ERBB2, ERBB3, and ERBB4, ERBB2 also called HER2 (Heregulin-2) and ERBB3 are closely related to the EGFR/ERBB1, but unlike EGFR, ERBB2 is a ligandless receptor, whereas ERBB3 lacks tyrosine kinase activity. Hence, both ERBB2 and ERBB3 are active only in the context of ERBB heterodimers, and ERBB2-ERBB3 heterodimers, which are driven by NRG (Neuregulin) ligands, are the most prevalent and potent complexes in terms of cell growth and transformation. The basis for the potency of signaling by the ligand-activated[..]
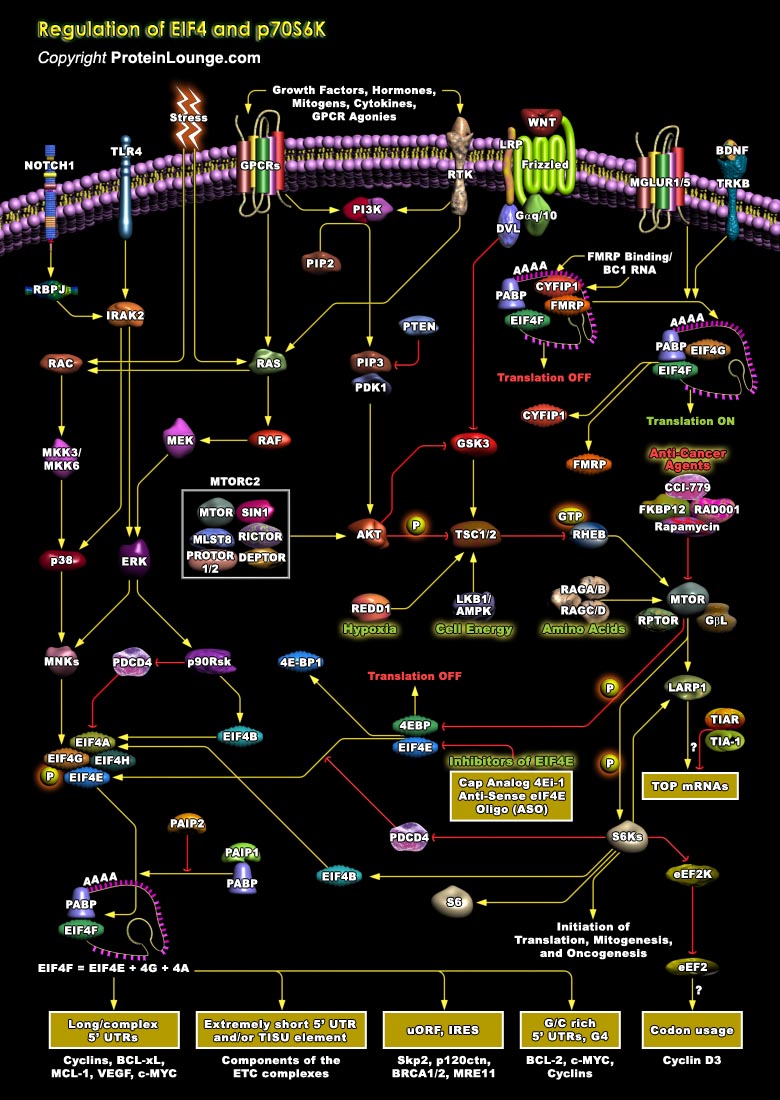
Protein synthesis regulation in eukaryotes is important for the modulation of gene expression. The process of mRNA translation/protein synthesis is generally initiated by eukaryotic initiation factors (eIFs), which along with p70S6K play critical roles in translational regulation (Ref.1). During mRNA translation, eukaryotic initiation factor 4E (eIF4E) (the m7GTP cap-binding protein) binds to eIF4G (a scaffolding subunit) and eIF4A (an ATP-dependent RNA helicase) to form active eIF4F complex. eIF4F complex binds to the 7-methyl-GTP cap structure present at the 5’ termini of all eukaryotic mRNAs and recruit the ribosome near the 5’ terminus of mRNA. PABP (Poly (A)-Binding Protein) also play an important role in the recruitment of mRNAs to ribosomes[..]
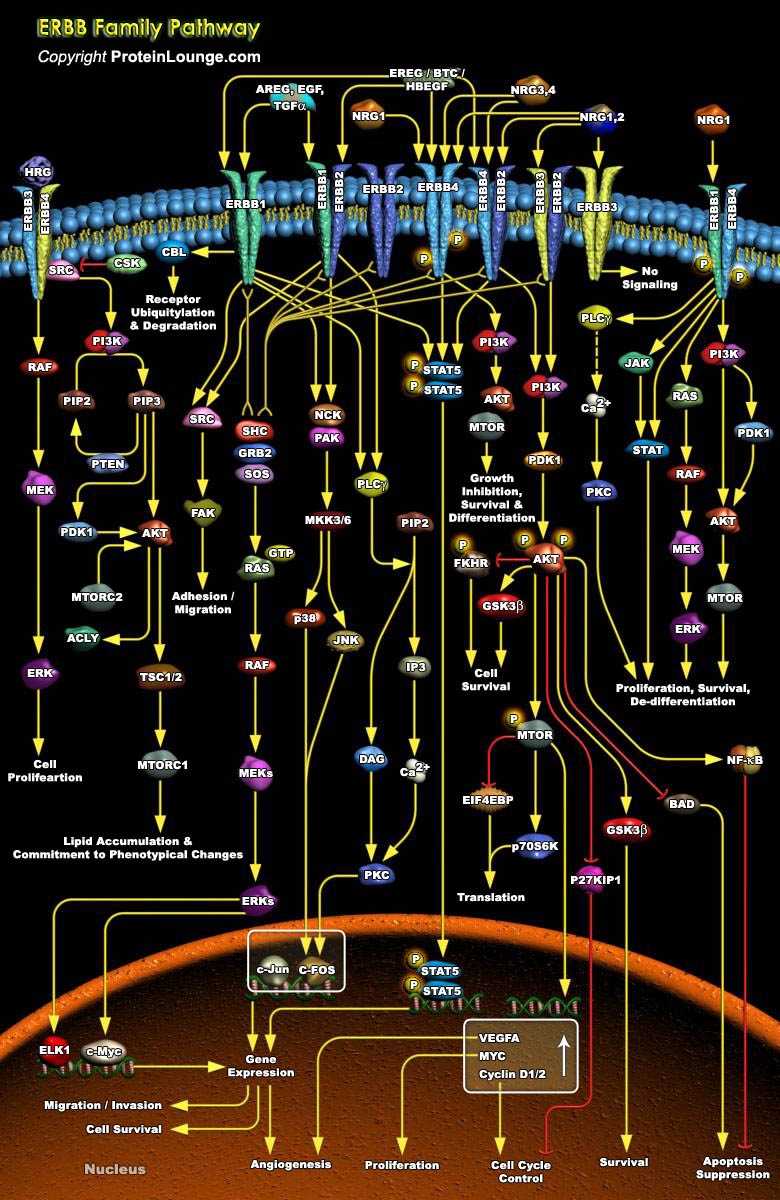
The ERBB (Erythroblastic Leukemia Viral Oncogene Homolog) or EGF (Epidermal Growth Factor) family of transmembrane RTKs (Receptor Tyrosine Kinases) plays an important role during the growth and development of a number of organs including the heart, the mammary gland, and the central nervous system. In addition, ERBB overexpression is associated with tumorigenesis of the breast, ovaries, brain, and prostate gland. The ERBB family includes four members, EGFR (EGF Receptor)/ERBB1/HER1 (Heregulin-1), ERBB2/HER2 (Heregulin-2), ERBB3/HER3 (Heregulin-3), and ERBB4/HER4 (Heregulin-4).Two of the family members, ERBB1 and ERBB2, are involved in the development of many types of human cancer. All ERBBs have in common an extracellular ligand-binding domain, a single[..]
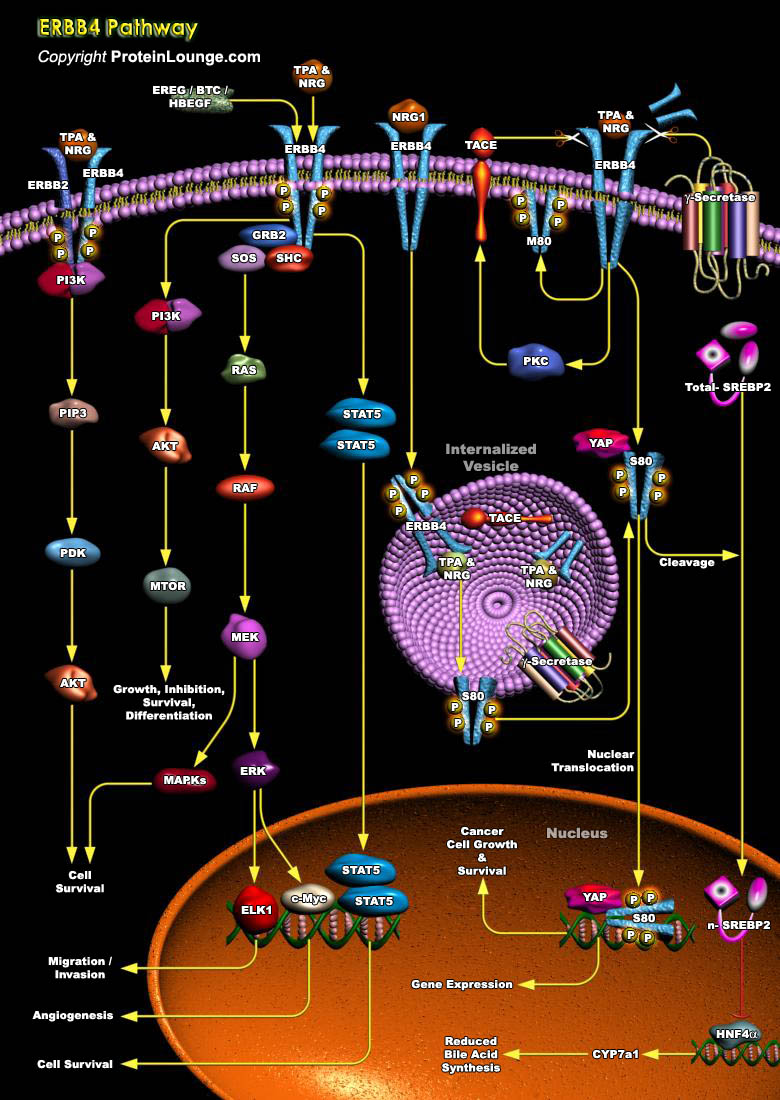
ERBB4 (Erythroblastic Leukemia Viral Oncogene Homolog-4) is a 180-kDa transmembrane RTK (Receptor Tyrosine Kinase) that regulates cell proliferation and differentiation. The ERBB4 is a member of the EGFR (epidermal growth factor receptor) subfamily of transmembrane RTKs. ERBB4 is expressed in several tissues, mainly heart, mammary gland and the central nervous system. ERBB4 and its ligands have important roles in normal cardiovascular and neural development, differentiation of the mammary gland, and in pathological conditions, such as heart diseases and cancer (Ref.1).ERBB4 can be activated by at least seven members of EGF-related peptide growth factors: betacellulin, epiregulin, HB-EGF (heparin-binding EGF-like growth factor) and the neuregulins (NRG-1, NRG-2, NRG-3,[..]

