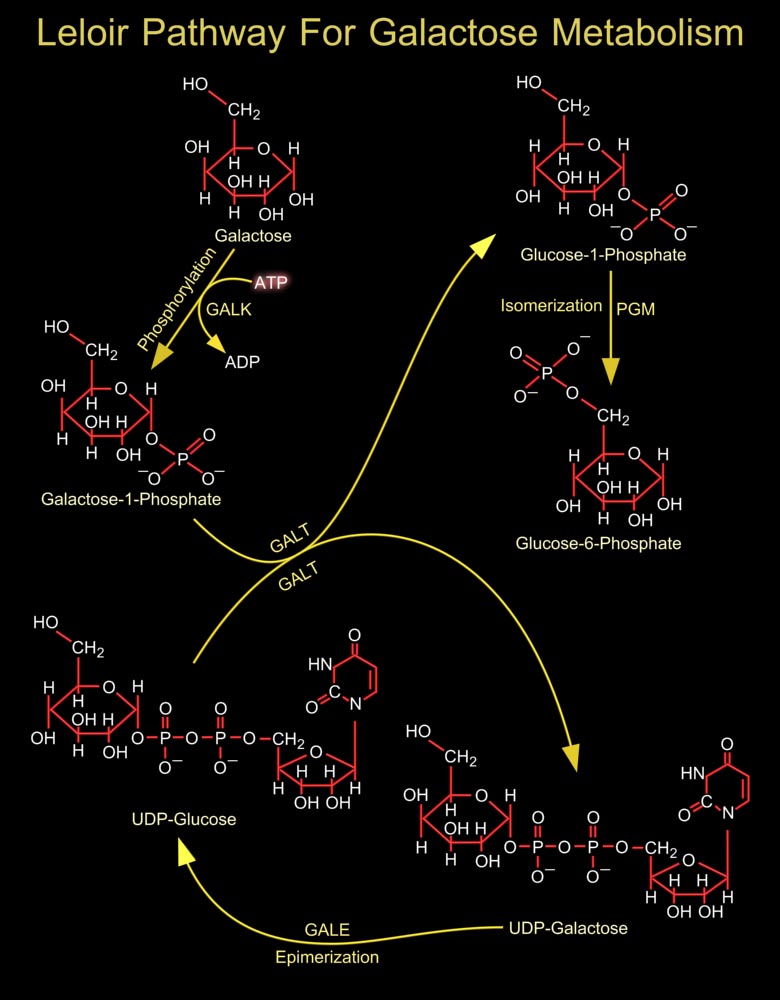
In most organisms, the conversion of Galactose to the more metabolically useful Glucose-1-Phosphate is accomplished by the action of four enzymes that constitute the Leloir pathway. In the first step of this pathway, Beta-D-Galactose is epimerized to Alpha-D-Galactose by GALM (Galactose Mutarotase/Aldose 1-Epimerase) (Ref.1). The active site of GALM is positioned in a rather open cleft with the hydroxyl groups of Galactose lying within hydrogen bonding distance to a number of side chains, the reaction catalyzed by human GALM proceeds via Glu-307 (Glutamic acid-307) and His-176 (Histidine-176). Till now no diseases have been attributed to mutations in human GALM. The next step involves the ATP-dependent phosphorylation of Alpha-D-Galactose by GalK (Galactokinase) to[..]
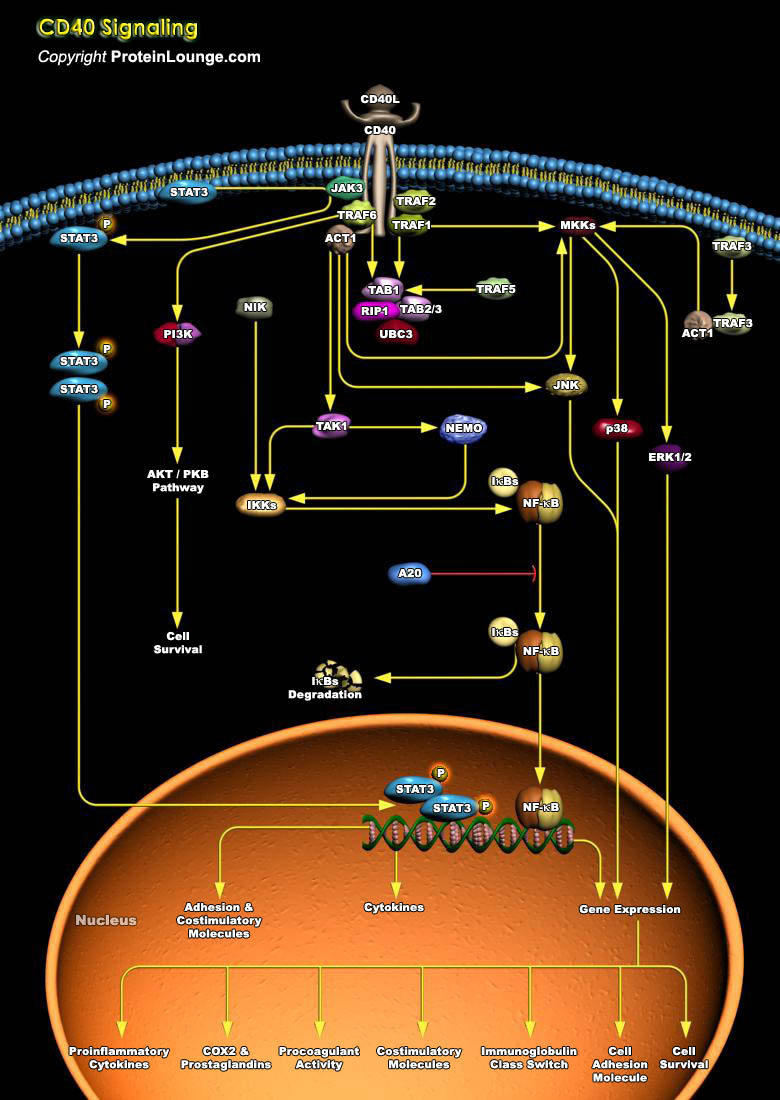
CD40, a TNFR (Tumor Necrosis Factor Receptor) family member, conveys signals regulating diverse cellular responses, ranging from proliferation and differentiation to growth suppression and cell death. First identified and functionally characterized on B-Cells, CD40 is expressed on a plethora of different cell types, including B-Cells, macrophages, dendritic cells, endothelial cells, and fibroblasts, and this widespread expression accounts for the central role of CD40 in the regulation of immune response and host defense (Ref.1). Binding of CD40 with its counter receptor, CD154 (also termed CD40L [CD40 ligand] or GP39), acts on Antigen presenting cells and T-Cells in a bi-directional fashion, mediating both humoral and cellular immune responses (Ref.2). Unique to[..]
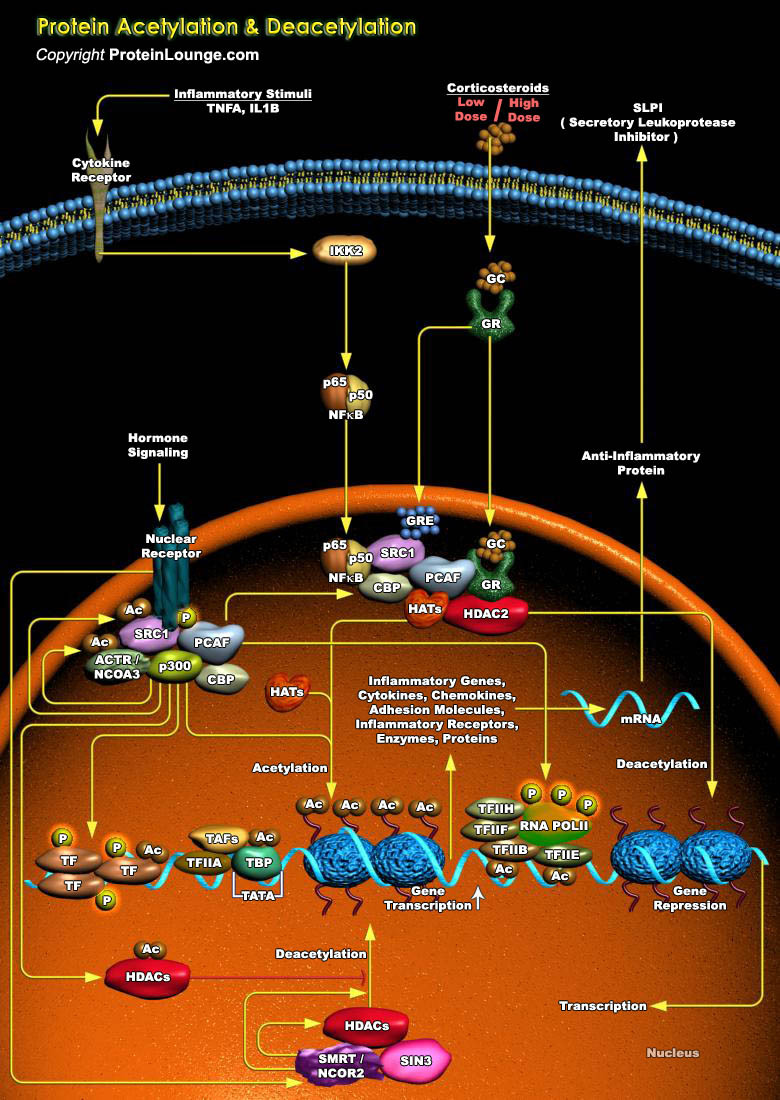
Eukaryotic transcription is a highly regulated process, and acetylation plays a major role in this regulation. Acetylation can occur on histones, DNA-binding TF (Transcription Factors), acetylases, nuclear import factors, non-nuclear proteins (Alpha-tubulin) and proteins that shuttle from the nucleus to the cytoplasm, such as the Importin-Alpha family of nuclear import factors. Acetylation can modify the recognition of DNA, the stability of proteins and the interaction between proteins. They regulate different cellular processes, such as microtubule function or nuclear import (Ref.1). By modifying chromatin proteins and transcription-related factors, these acetylases regulate diverse functions, including DNA recognition, protein-protein interaction, protein stability,[..]

The DNA damage response network involves several kinases to inactivate CDK/ cyclin complexes and result in cell cycle arrest for DNA repair. In the presence of DNA damage or incomplete DNA replication, eukaryotic cells activate cell cycle checkpoints that temporarily halt the cell cycle to permit DNA repair or completion of DNA replication to take place. In the presence of extensive damage or absence of timely repair, these checkpoint-signaling pathways may also trigger a pathway that effects programmed cell death or apoptosis. DNA damage-activated cell cycle checkpoints are regulated in part by the phosphoinositide kinase family of checkpoint components, including the yeast Rad3 in Schizosaccharomyces pombe, Mec1/Tel1 in Saccharomyces cerevisiae, mammalian ATM (Ataxia[..]
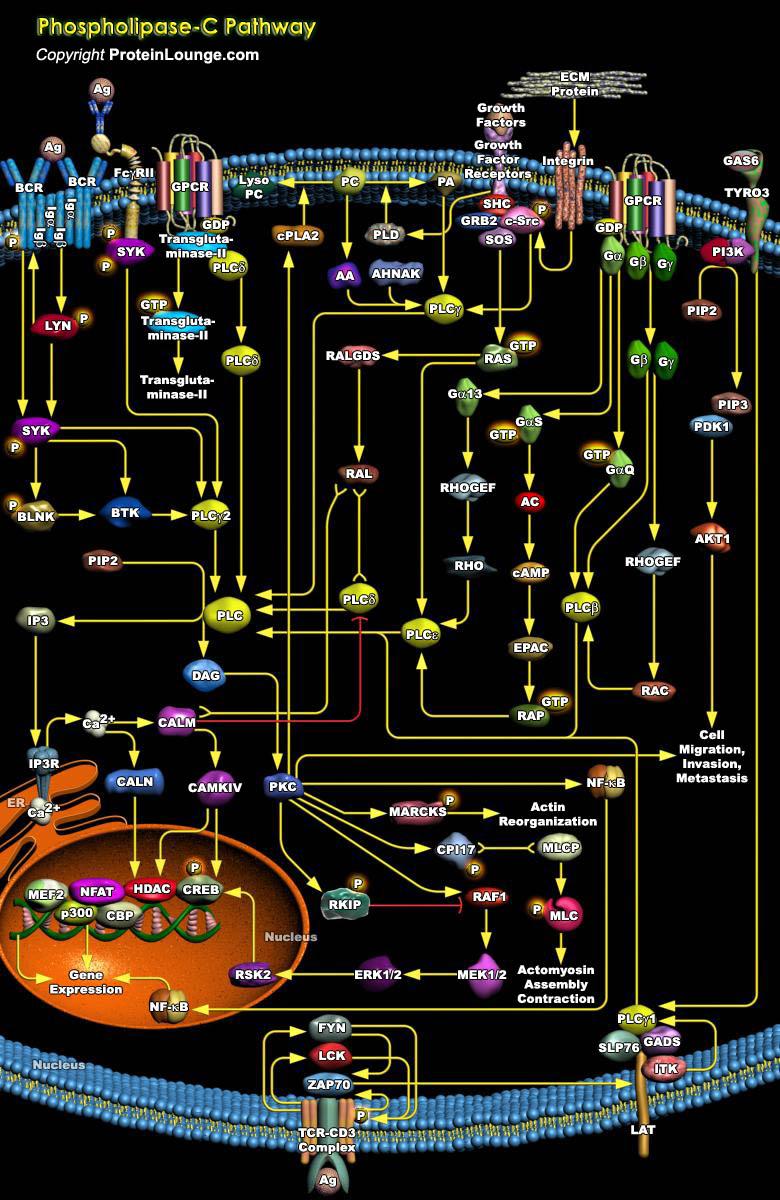
Inositol lipid-specific PLC (Phospholipase-C) isozymes are key signaling proteins in the cellular action of many hormones, neurotransmitters, growth factors, and other extracellular stimuli. PLC are soluble proteins that are partly cytosolic and partly associated with membrane. The PLC family in human is comprised of 13 subtypes. On the basis of their structure, they have been divided into six classes, PLC-Beta (Beta 1, 2, 3 and 4), PLC-Gamma (Gamma 1 and 2), PLC-Delta (Delta 1, 3 and 4), PLC-Epsilon, PLC-Zeta and PLC-Eta (Eta1 and 2) types. The molecular weights of each are 85kDa for the Delta form, 120-155kDa for both the Beta and Gamma forms, and 230-260kDa for the Epsilon form. These groups also differ in the mechanisms by which the isozymes are activated in[..]
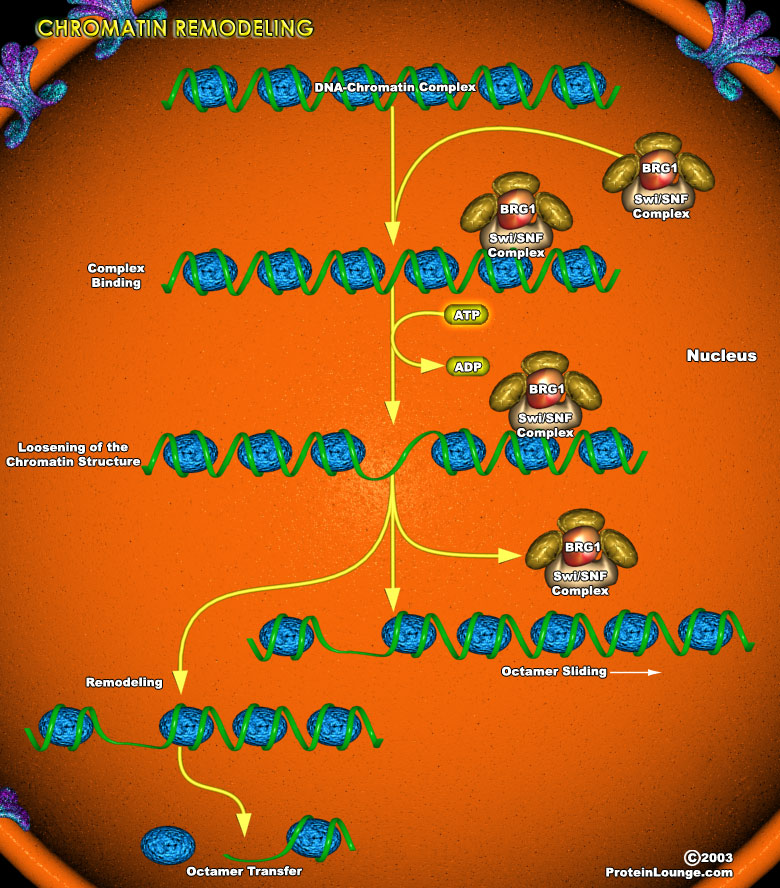
The condensation of DNA into an ordered chromatin structure allows the cell to solve the topological problems associated with storing huge molecules of chromosomal DNA within the nucleus. DNA is packaged into chromatin in orderly repeating protein-DNA complexes called nucleosomes. Each nucleosome consists of approximately 146bp of dsDNA (double-stranded DNA) wound 1.8 times around a histone octamer.Two molecules each of H2A, H2B, H3, and H4 comprise the histone ramp around which the DNA superhelix winds. Stretches of DNA upto 100bp separate adjacent nucleosomes. Multiple nuclear proteins bind to this linker region, some of which may be responsible for the ordered wrapping of strings of nucleosomes into higher-order chromatin structures (Ref.1).Chromatin assembly[..]
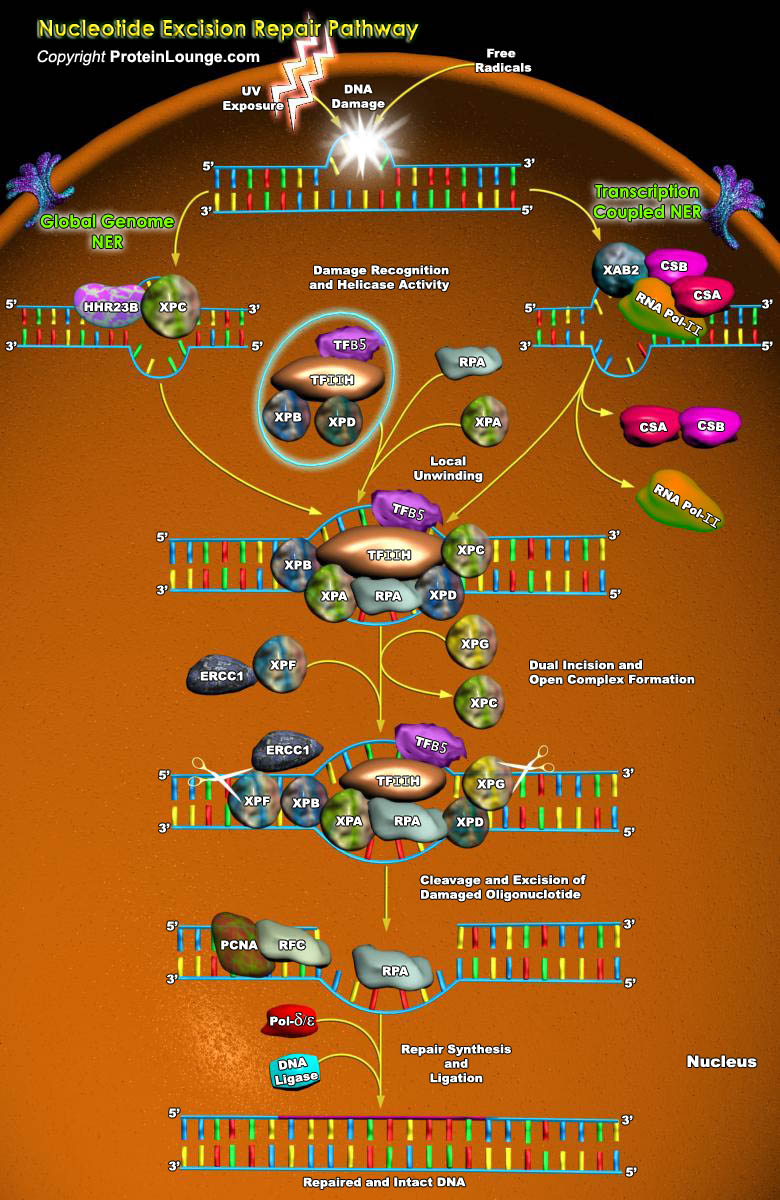
Nucleotide excision repair (NER) is the main pathway used by mammals to remove bulky DNA lesions such as those formed by UV light, environmental mutagens, and some cancer chemotherapeutic adducts from DNA. Deficiencies in NER are associated with the extremely skin cancer-prone inherited disorder xeroderma pigmentosum. Although the core NER reaction and the factors that execute it have been known for some years, recent studies have led to a much more detailed understanding of the NER mechanism, how NER operates in the context of chromatin, and how it is connected to other cellular processes such as DNA damage signaling and transcription(Ref.1). The NER process requires the action of more than 30 proteins in a stepwise manner that includes damage recognition, local[..]
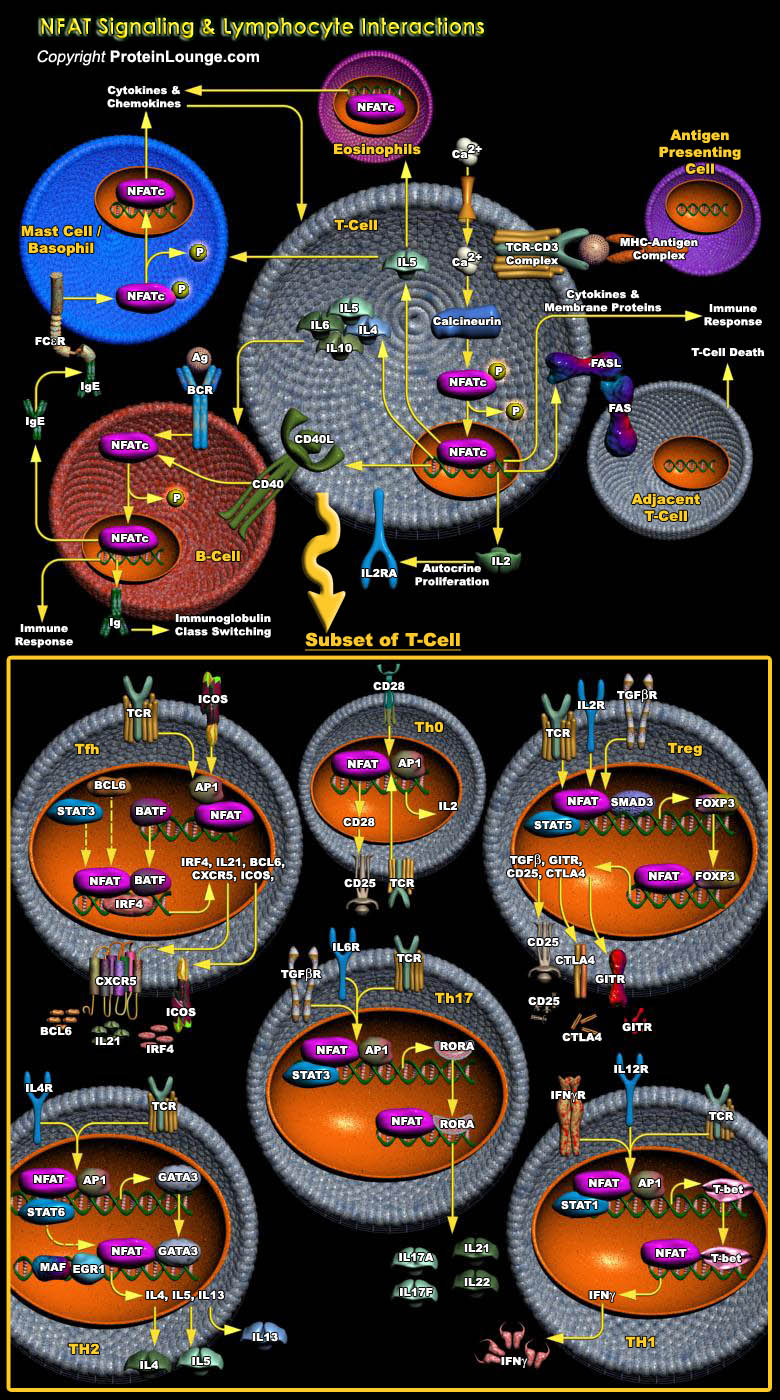
The optimum functioning of the immune system is crucial for human survival. The invading pathogens are encountered by the cells of the immune system, which include T-Cells, B-Cells, macrophages, neutrophils, basophils, eosinophils, endothelial cells, or mast cells. These cells have distinct roles in the immune system, and cell-to-cell communication among these cells is an indispensable prerequisite for the stimulation of the optimum immune response. In the process, cytokines serve as signal molecules for communication that are induced by several cell-signaling cascades. NFATs (Nuclear factors of activated T-Cells), a family of transcription factors expressed by diverse cell types of the immune system plays a pivotal role in the process. Originally described in[..]
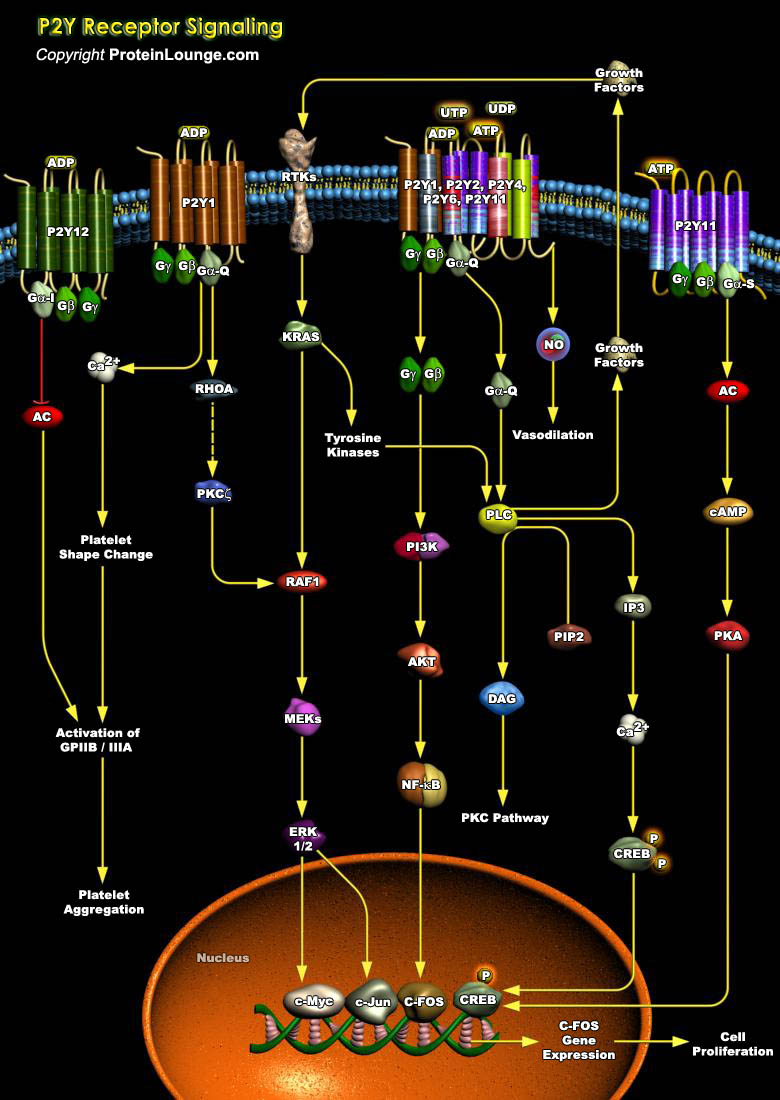
Angiogenesis plays an important role in pathological events such as tumor growth, wound healing, psoriasis, and the ischemic retinopathies that occur in diabetes and sickle cell disease. The main modulators of the angiogenesis process in adults, is achieved through signaling by peptide growth factors, however, recent researches also emphasize the contribution of purines and pyrimidines to this process. Synergistic actions of purines and pyrimidines with growth factors aid in promoting cell proliferation (Ref.1). ATP (Adenosine Triphosphate), ADP (Adenosine Diphosphate), UTP (Uridine Triphosphate), UDP (Uridine Diphosphate) and adenosine play pivotal signaling roles in these long-term events, referred to as purinergic signaling, mediated via P1 and P2 receptors.[..]
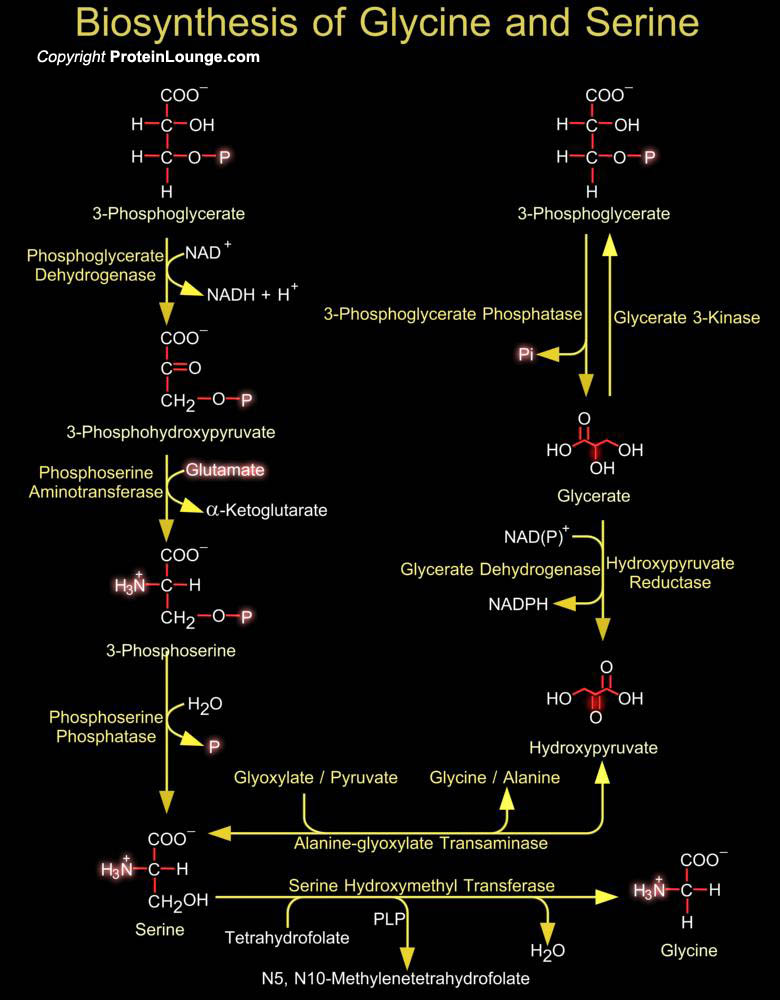
Intermediates in energy production pathways such as glycolysis and the Krebs cycle are commonly the starting point for the biosynthesis of amino acids. The biosynthesis of serine and glycine constitute a major metabolic pathway that plays a central role in the formation of other amino acids, nucleic acids and phospholipids. Serine an ɑ-amino acid used in the biosynthesis of proteins can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It contains an α-amino group a carboxyl group, and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. Glycine is also an amino acid that with a single hydrogen atom as its side chain. It is one of the proteinogenic amino acids and[..]
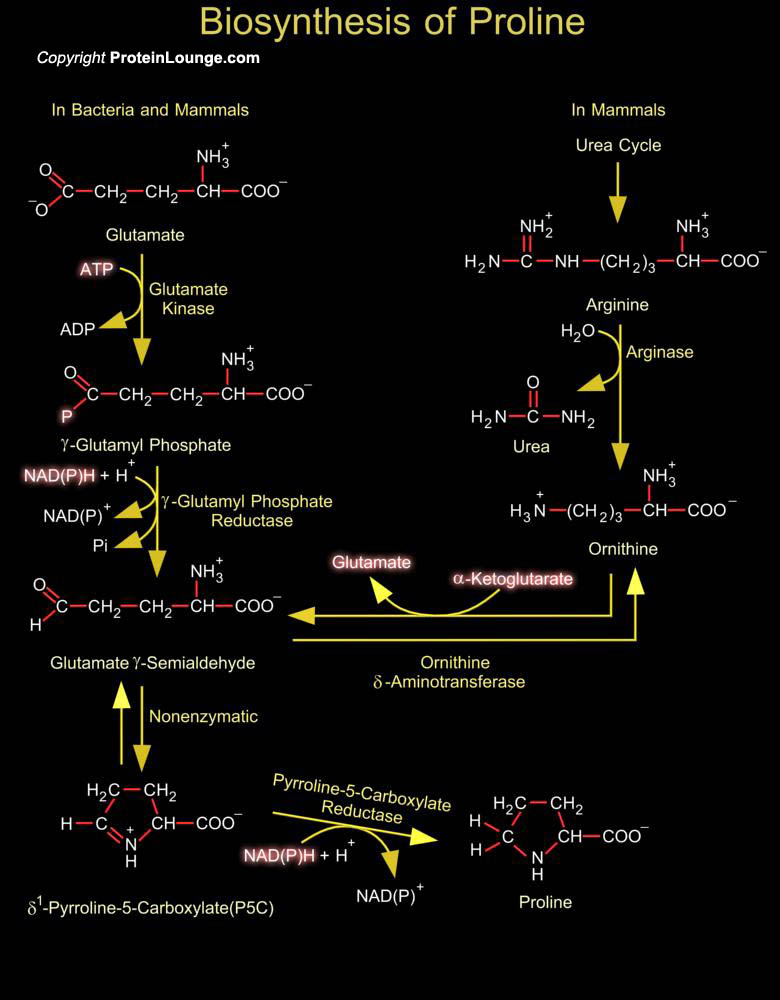
Proline is unique in having a cyclic structure with its side chain connected to the amino group to create a secondary amine. As a consequence of its cyclic structure, Proline constrains the structure of proteins where it occurs, disrupting Alpha-helices. Isomerization between the cis and trans forms of Proline in proteins is isomerized by peptidyl-prolyl isomerases like the cyclophilins that contribute to protein folding and are components of signal transduction pathways (Ref.1). Proline is derived from glutamate. Its biosynthesis begins with the ATP-driven phosphorylation and reduction of the carboxyl side chain of glutamate. The Gamma-carboxylate group of glutamate is activated by phosphorylation with ATP to from a Gamma-Glutamyl Phosphate intermediate. The[..]
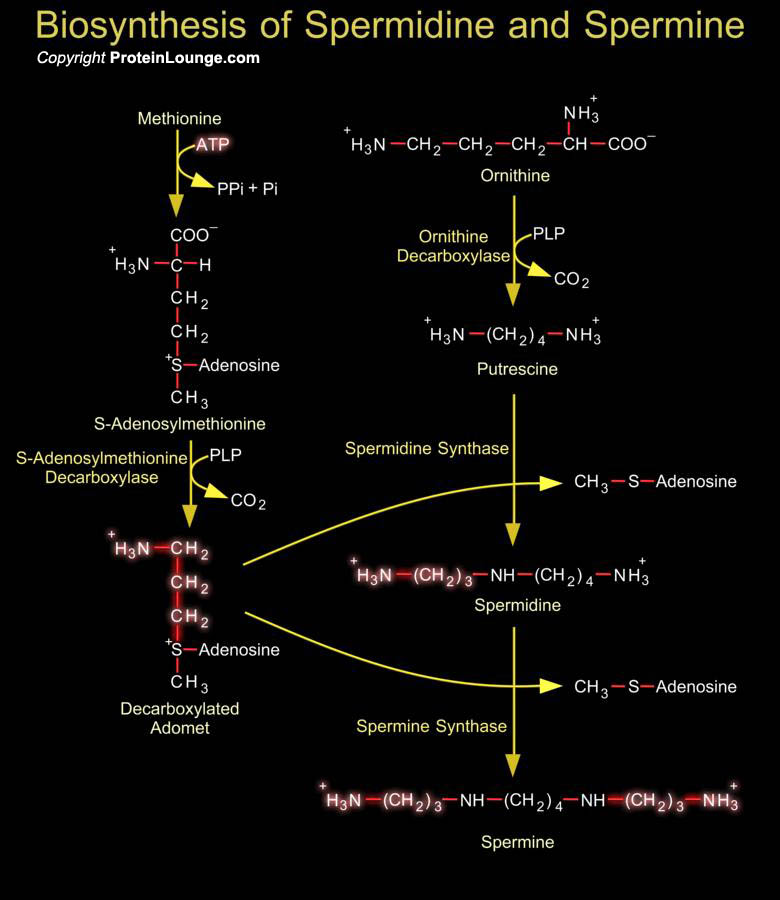
The polyamines Spermidine and Spermine are naturally occurring ubiquitous polycations involved in the regulation of cell growth, differentiation, apoptosis and progression through the cell cycle. (Ref.1). Cellular polyamine levels are regulated by multiple pathways such as synthesis from amino acid precursors, cellular uptake mechanisms that salvage polyamines from diet and intestinal microorganisms, as well as stepwise degradation and efflux (Ref.2). Putrescine and S-Adenosylmethionine are the obligate precursors for Spermidine and Spermine. In mammals Putrescine is formed by decarboxylation of Ornithine by the action of Ornithine Decarboxylase. Ornithine, one of the starting materials for polyamine biosynthesis, is derived from the amino acid Arginine as part of[..]

