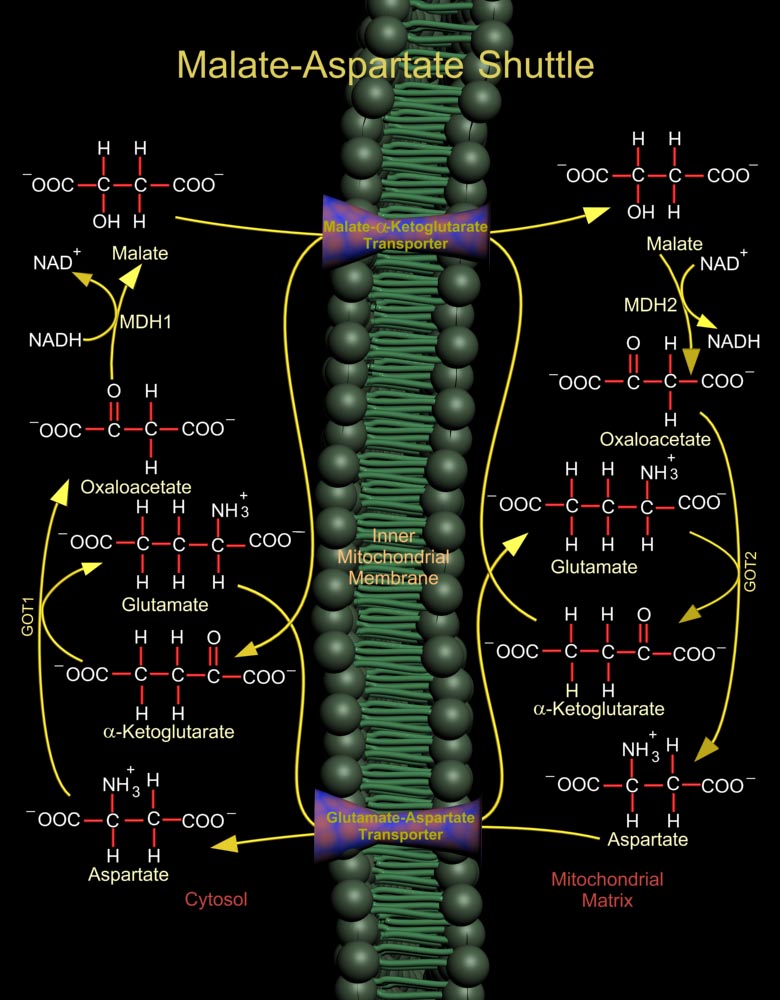
The Malate (L-Malic Acid)-Asp (Aspartate or L-Aspartate or Aspartic Acid) Shuttle of mammalian systems is more complex but more energy efficient. Mitochondrial NAD+ (Nicotinamide Adenine Dinucleotide) is reduced by cytosolic NADH (Nicotinamide Adenine Dinucleotide, Reduced) through the intermediate reduction and subsequent regeneration of OAA (Oxaloacetate) (Ref.1). In the cytosol, the shuttle converts OAA to Malate using the enzyme MDH1 (Malate Dehydrogenase-Cytoplasmic), and at the same time re-oxidizes NADH to NAD+, making it available for Glucose oxidation. Malate is then shuttled into the mitochondria by a Malate-Alpha-Ketoglutarate Transporter. In the mitochondria, the reverse reaction takes place and Malate is again converted to OAA by the enzyme MDH2 (Malate[..]
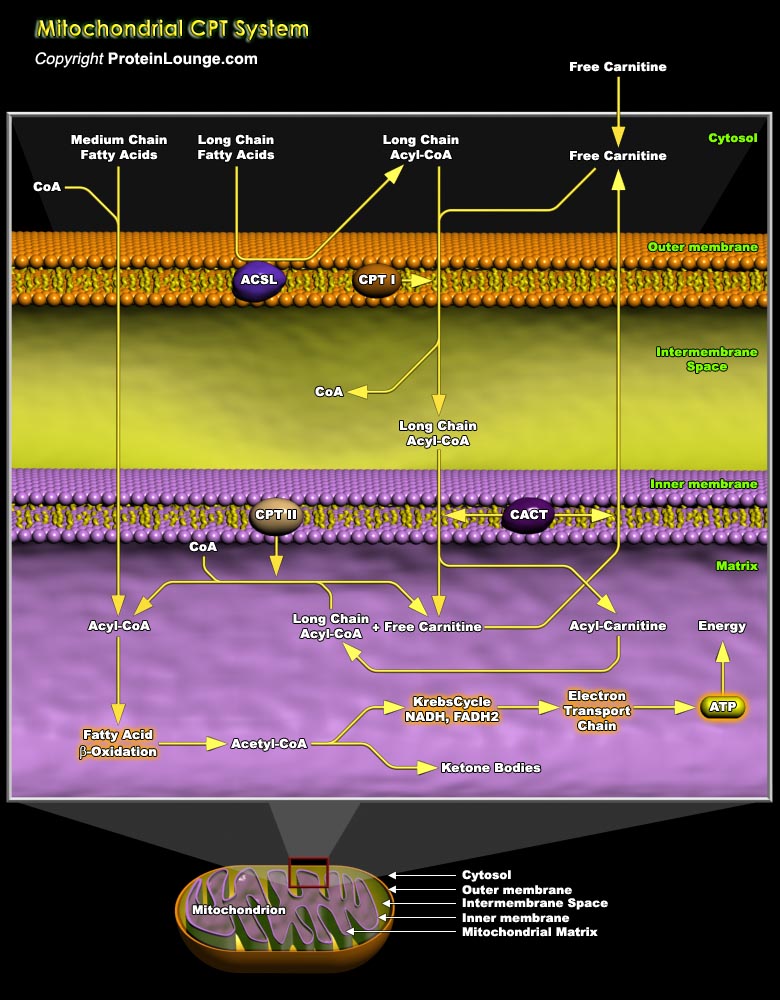
Mitochondrial fatty acid oxidation (FAO) and its key rate-limiting enzyme, the carnitine palmitoyltransferase (CPT) system, are important for energy homeostasis in situations like fasting or during exercise. They also regulate host immune responses and the deficiency or over-activation of CPT may cause energy metabolism disorder that may lead to many diseases starting from inflammatory disorders to cancer. Fatty acid catabolism occurs mostly in mitochondria through the beta-oxidation pathway (Ref.1 and 2). Long-chain fatty acids (LCFA), major component of fatty acids, that are important source of energy for heart, liver and muscles, cannot enter the mitochondria by simple diffusion unlike short or medium chain fatty acids. LCFA are activated and converted to[..]
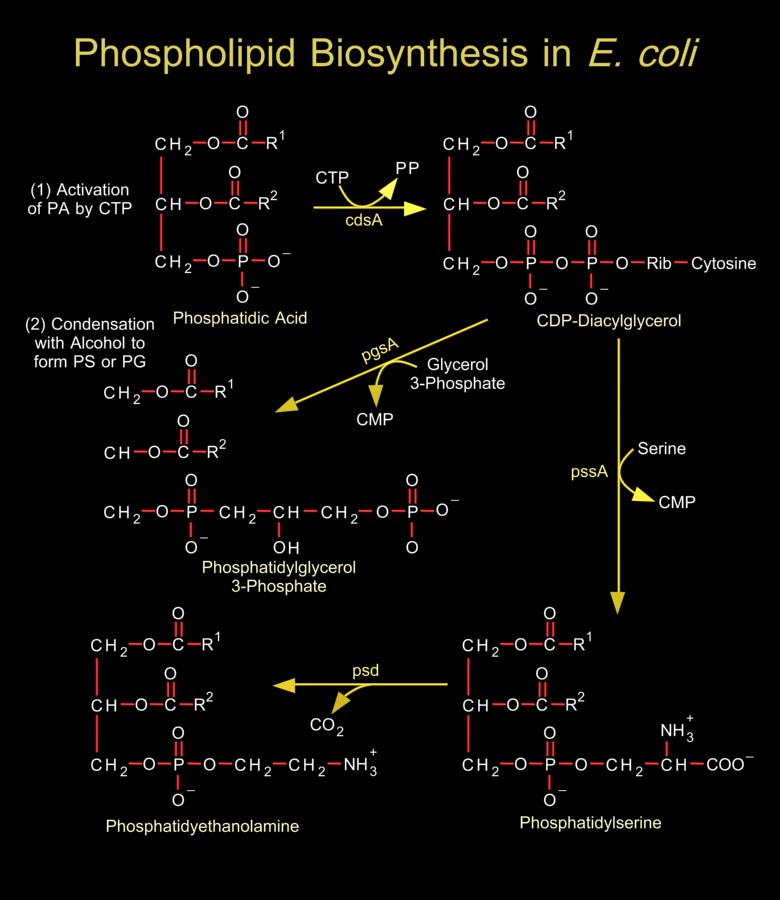
Biological membranes are composed of hundreds of distinct proteins and phospholipids. Phospholipids are diacylglycerol derivatives with a hydrophilic, zwitter ionic, often charged headgroup at position C3 of the glycerol backbone. The properties of phospholipids give lipid bilayer membranes their self-organizing structure. Phospholipids are usually composed of two fatty acid chains esterified to two of the carbons of glycerol phosphate, the phosphate being esterified to a hydroxyl group of another hydrophilic compound, such as choline, ethanolamine or serine.In general, E. coli and most related gram-negative bacteria do not contain phosphatidylcholine, phosphatidylinositol, sphingolipids, glycolipids, plasmalogens, or sterols, which are characteristic of[..]
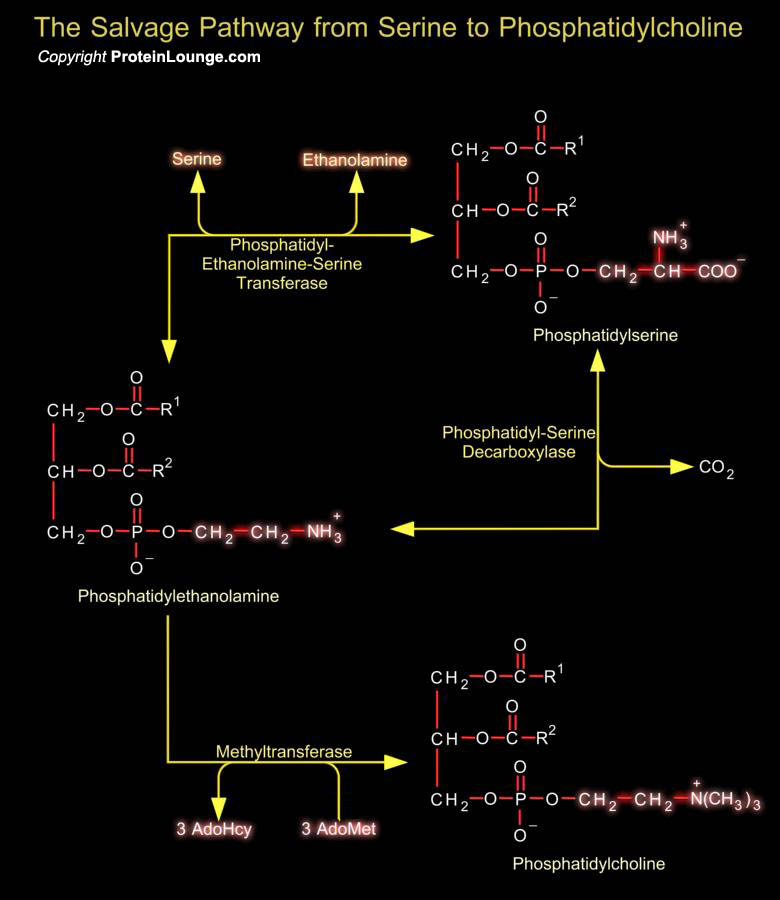
Phosphatidylserine (or 1, 2-Diacyl-sn-Glycero-3-Phospho-L-Serine) is the only amino acid-containing glycerophospholipid in animal cells. Although it is distributed widely among animals, plants and microorganisms, it is usually less than 10% of the total phospholipids, the greatest concentration being in myelin from brain tissue. It is an acidic (anionic) phospholipid with three ionizable groups, i.e. the phosphate moiety, the amino group and the carboxyl function. As with other acidic lipids, it exists in nature in salt form.In bacteria and other prokaryotic organisms, Phosphatidylserine is synthesized by a mechanism comparable to that of other phospholipids, i.e. by reaction of L-Serine with CDP-DAG (CDP-Diacylglycerol).In contrast in animal tissues, there are two[..]
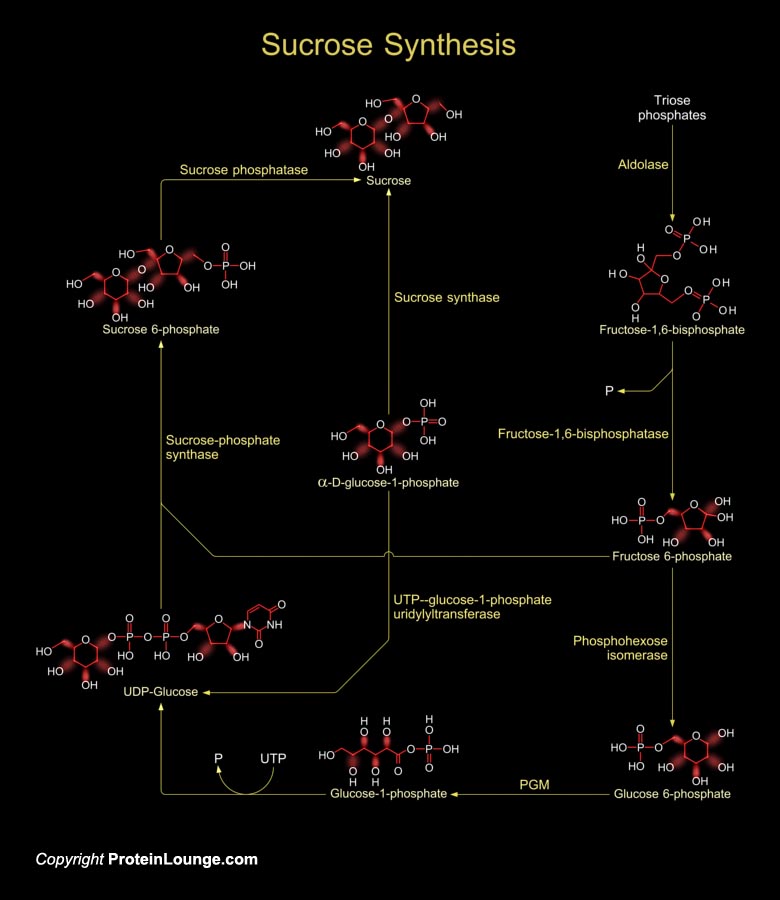
Sucrose is produced by plants, algae and cyanobacteria as an end product of photosynthesis. It is the primary sugar transported from the source tissues to sink tissues by the phloem in most plants. In other non-photosynthetic tissues, sucrose serve as raw material for many metabolic pathways (Ref.1).Sucrose synthesis starts in the cytosol where two triose-phosphates produce one Fructose-1,6-bisphosphate by the enzyme aldolase. Fructose-1,6-bisphosphate is converted to fructose-6-phosphate by the enzyme Fructose-1,6-bisphosphatase. Then the enzyme phosphohexose isomerase converts fructose-6 phosphate to glucose-6-phosphate. Glucose-6-phosphate then forms glucose-1-phosphate in presence of the enzyme phosphoglucomutase (PGM). The glucose-1-phosphate is converted to[..]
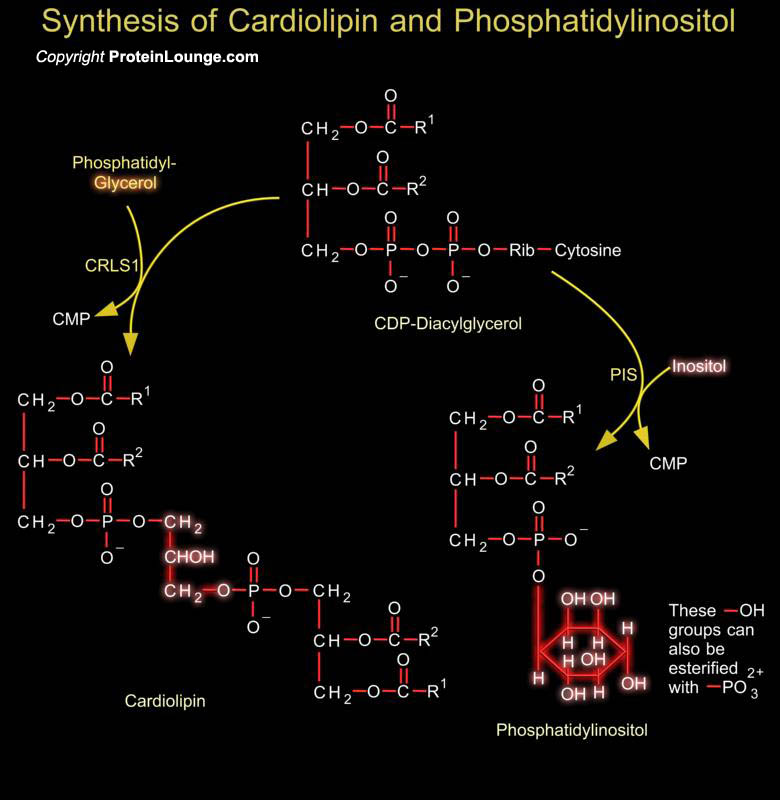
Phosphoglycerides such as phosphatidylethanolamine and phosphatidylserine are the major component of lipid bilayer membranes in which their detergent-like properties help to provide membranes the quality of self-assembly. Phosphatidylglycerol is a precursor in the synthesis of both cardiolipin and PI (Phosphatidylinositol). Cardiolipin is a unique dimeric phospholipid found in the heart and most mammalian tissues. This phospholipid is the only phospholipid localized primarily in the mitochondrial membrane of mammalian cells. In cardiolipin, two phosphatidylglycerol units are joined together. Phosphatidic acid is activated to CDP-DAG (CDP-Diacylglycerol), which is the precursor to PI and cardiolipin. PI is the precursor of PI Phosphates, which have one of more[..]
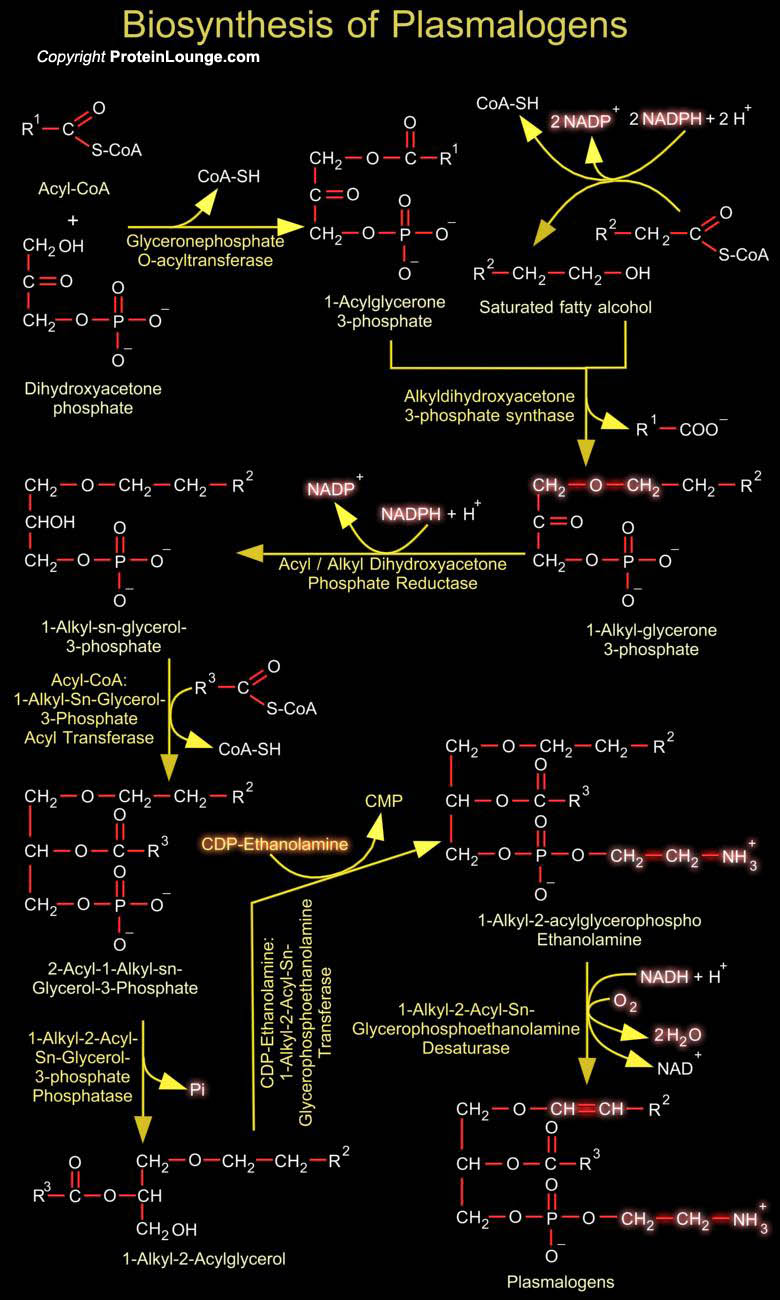
Plasmalogens are glycerophospholipids of neural membranes containing vinyl ether bonds. Their synthetic pathway is located in peroxisomes and endoplasmic reticulum. About 20% of mammalian Glycerophospholipids are Plasmalogens. The exact percentage varies both from species to species and from tissue to tissue within a given organisms. While Plamalogens comprise only 0.8% of phospholipids in human liver, they account for 23% of those in human nervous tissue (Ref.1). One of the starting materials for plasmalogen biosynthesis is DHAP (Dihydroxyacetone Phosphate) from glycolysis, which is used to form the glycerol backbone of the plasmalogen. The initial steps of plasmalogen biosynthesis are catalyzed by DHAP-AT (Dihydroxyacetone phosphate Acyltransferase) and[..]
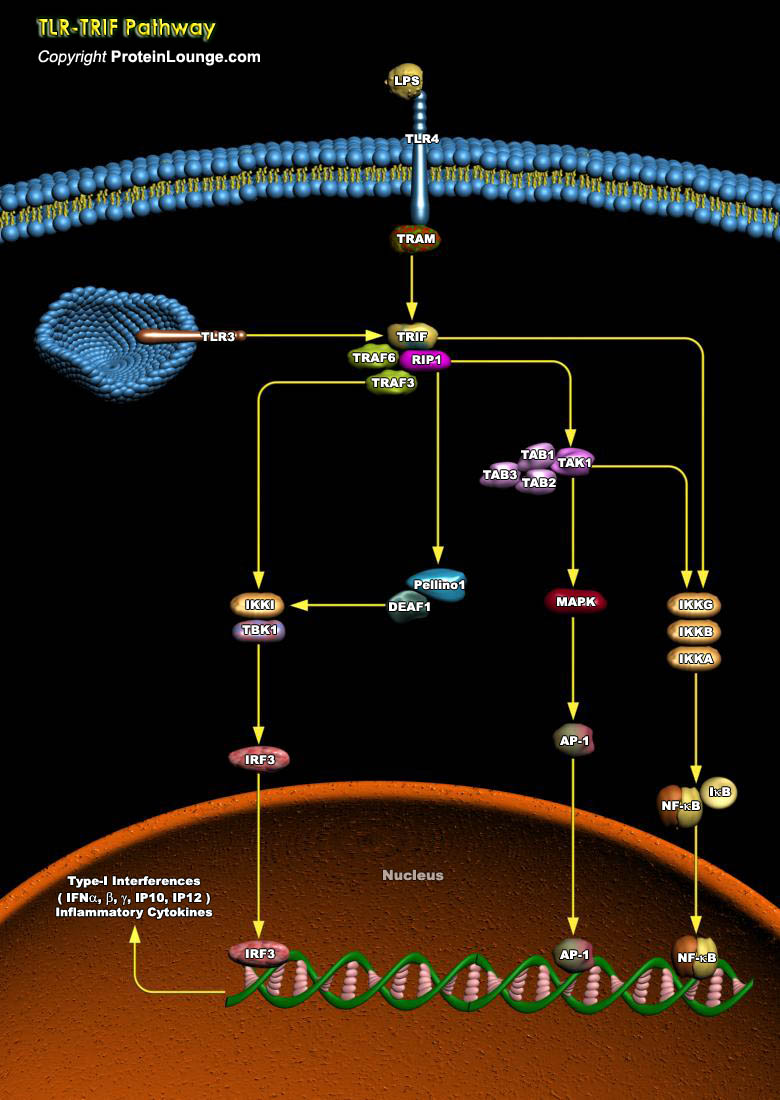
The Toll-like receptors (TLRs), 13 types known to-date, are a group of pattern-recognition receptors that play a crucial role in danger recognition and induction of the innate immune response against bacterial and viral infections. The major adaptors that bind to the intracellular domain of TLR to activate the proinflammatory response are the myeloid differentiation primary response MYD88 and TIR-domain-containing adapter-inducing interferon-beta (TRIF). TRIF-dependent signaling is required for TLR-mediated production of type-I IFN and several other proinflammatory mediators (Ref.1 and 2). The TLR adaptor molecule facilitates TLR3 and TLR4 signaling and concomitant activation of the transcription factors, NF-kappaB, AP-1 and IFN regulatory factor 3, leading to[..]
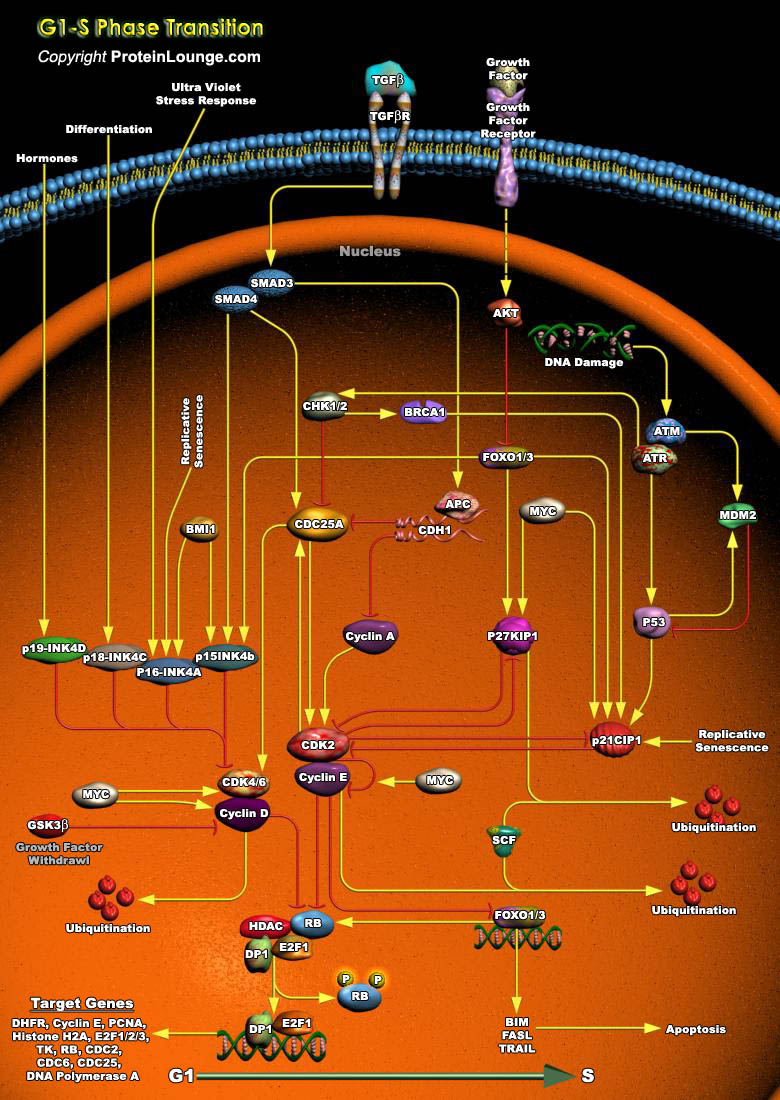
Cell cycle arrest in response to DNA damage is an important mechanism for maintaining genomic integrity. This cell cycle arrest provides time for DNA repair to prevent replication or segregation of damaged DNA. Induction of growth arrest by DNA damage occurs mainly through the activation of checkpoint pathways that delay cell cycle progression at G1, S, and G2 (Ref.1 and 2).In vertebrate cells, the commitment to complete a round of mitotic division takes place during the initial phase of the cell cycle (G1), at a stage called the restriction (R) point, preceding the onset of DNA synthesis (S-phase).The primary G1/S cell cycle checkpoint controls the commitment of eukaryotic cells to transition through the G1 phase to enter into the DNA synthesis S phase. Two cell cycle[..]
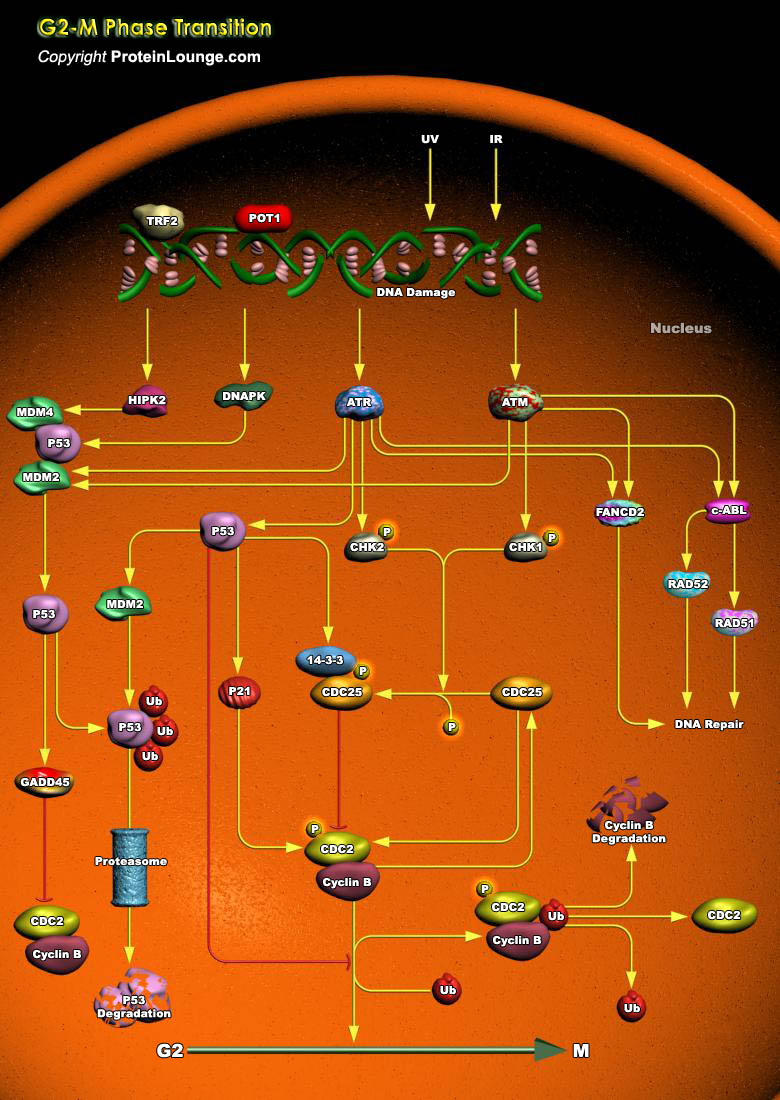
The cellular responses to DNA damage induced by ionizing radiation include activation of cell cycle checkpoints that delay progression of cells through the cell cycle. Ionizing radiation-induced checkpoints are active at the transition from the G1-phase to the S-phase, in the S-phase, and at the transition from the G2-phase to mitosis (G2/M). The surveillance mechanisms responsible for initiating these checkpoints appear to facilitate maintenance of the integrity of the genome, presumably because they ensure that damaged DNA templates are neither replicated nor segregated into the daughter cells until they are repaired. The option to undergo apoptosis can be considered as one of the checkpoint endpoints, and a failure of the cell to die under appropriate circumstances[..]
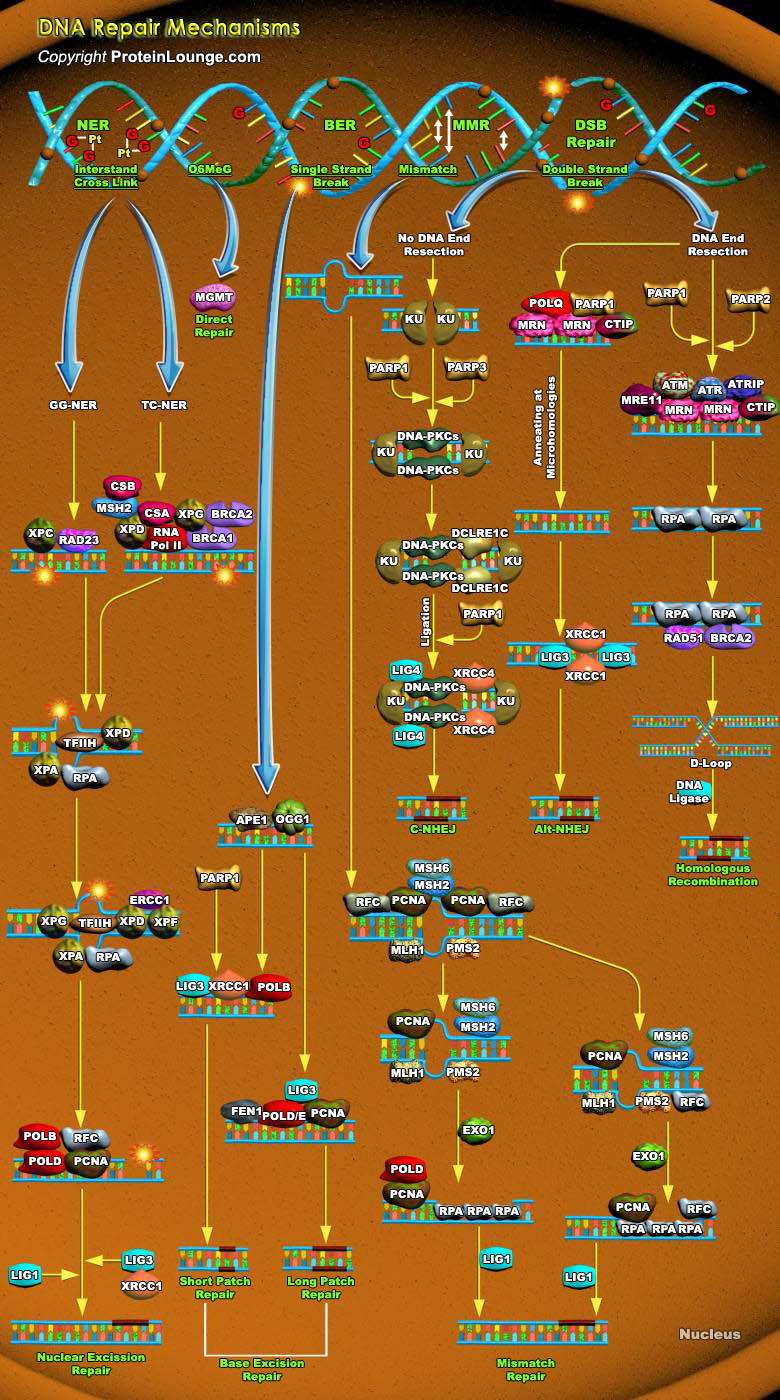
Cells are constantly under threat from the cytotoxic and mutagenic effects of DNA damaging agents that result from either endogenous sources (cellular metabolic processes) or exogenous sources (environmental factors). Endogenous sources of DNA damage include hydrolysis, oxidation, alkylation, and mismatch of DNA bases; sources for exogenous DNA damage include ionizing radiation (IR), ultraviolet (UV) radiation, and various chemicals agents (Ref.1). Repairing damage in DNA from anything that causes a mutation, such as UV radiation and tobacco smoke, is a fundamental process that protects our cells from becoming cancerous. The major forms of DNA damage include SSB (Single-strand Breaks), DSB (Double-strand Breaks), alteration of bases, hydrolytic depurination, hydrolytic[..]
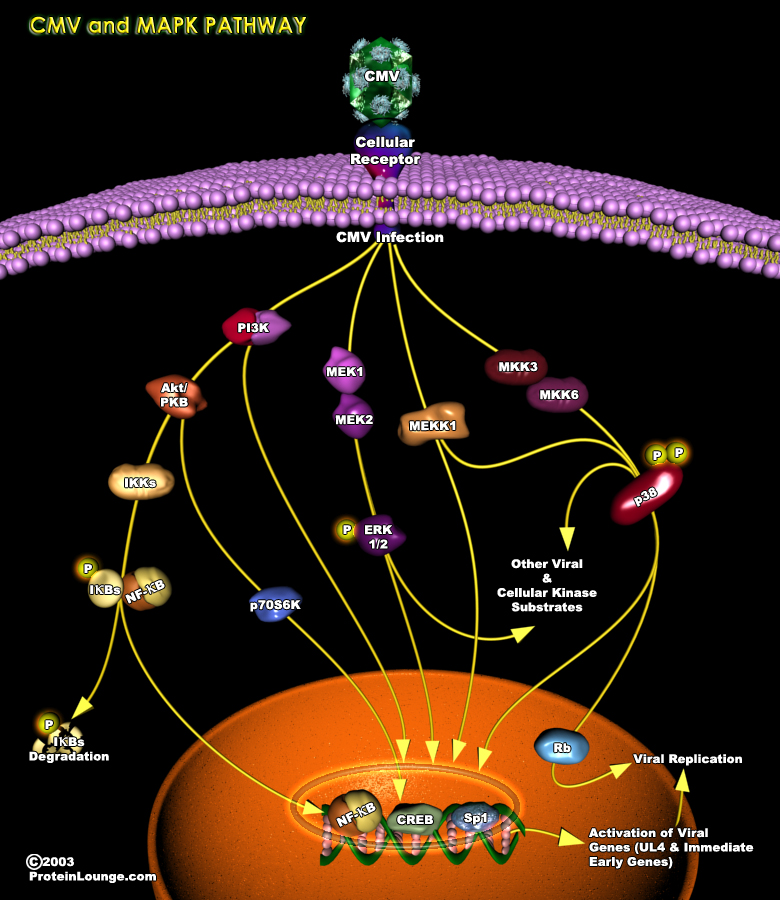
An upregulation of cellular signaling pathways is observed in multiple cell types upon HCMV(human cytomegalovirus) infection, suggesting that a global feature of HCMV infection is the activation of the host cell. HCMV initiates and maintains cellular signaling through a multitiered process that is dependent on a series of events: (1) the viral glycoprotein ligand interacts with its cognate receptor, (2) cellular enzymes and viral tegument proteins present in the incoming virion are released and (3) a variety of viral gene products are expressed. Viral-mediated cellular modification has differential outcomes depending on the cell type infected.Human CMV infects a significant portion of the human population and belongs to the family of beta-herpes virus(Ref.1&2).One[..]

